
Summary of the best evidence for the prevention and management of myasthenic crisis after thymectomy
Highlight box
Key findings
• A multidisciplinary, collaborative multidisciplinary team discussion should be conducted before surgery to evaluate the patient’s systemic condition, immune status, and cardiopulmonary function.
• Avoid neuromuscular blocking agent as much as possible, if this is required, we recommend using rocuronium bromide or vecuronium bromide and then reversing with sugammadex.
• Surgery should be scheduled as early in the day as possible, when the patient’s muscle strength is at its best.
• After surgery, respiratory function, coughing ability, and causes of sputum disturbance should be evaluated.
What was recommended and what is new?
• In previous versions, only the importance of intraoperative precautions and medication management was described.
• In this version, it is stated that respiratory management is also important for preventing and managing postoperative myasthenic crisis (MC).
What is the implication, and what should change now?
• The guideline includes prevention and management of MC during the entire perioperative period.
• The new recommendations in the guideline are conducive to the promotion of the concept of prevention and management of MC in clinical practice.
Introduction
The thymus plays an important immunological role in the development of myasthenia gravis (MG) (1). For this reason in about 80% of MG patients with thymic abnormalities, thymectomy has a therapeutic role (2). With the development and progress of minimally invasive surgical techniques, thoracoscopic and robot-assisted thymectomy have shown to be better accepted and resulted in reduced surgical trauma and shorter hospital stay than equivalent procedures performed by open surgery. However, the recovery of muscle strength after surgery may require long time and some patients may develop myasthenic crisis (MC) postoperatively with an incidence ranging between 6.2% and 30.3% (3,4). MC is mainly characterized by the sudden exacerbation of muscle weakness, especially in the intercostal, diaphragmatic, and pharyngeal muscles leading to reduced functional lung capacity, breathing difficulties, and increased throat and bronchial secretions that cannot be easily expectorated requiring non-invasive ventilation support or emergency re-intubation (5). Postoperative MC is reversible and preventive interventions are crucial to successfully manage potential harmful outcomes in operated patients. Previous clinical studies on postoperative MC were diversified, and most clinical staff relied on their own clinical experience without an overall framework. Summarizing the best evidence for the prevention and management of postoperative MC is an urgent need to guide clinical work. This study systematically searched for domestic and foreign evidence on the prevention and management of postoperative MC and integrated it to ultimately form the best evidence to provide a reference and guidelines for the formulation and standardization of measures for the prevention and management of postoperative MC.
Methods
Question identification
The PIPOST analysis method was used to construct the following evidence-based nursing problem: P (population evidence application group): MG patients after thymectomy; I (intervention): evaluations related to the occurrence of postoperative MC, drug/non-drug management, management of MC, etc.; P (professional implementor): clinical medical staff; O (outcome): the occurrence of MC, the medical staff’s knowledge of the disease and patient compliance; S (setting for evidence application): thoracic surgery ward, mediastinal surgery ward, intensive care unit; T (type of evidence): clinical guidelines, expert consensus, clinical decision-making, systematic evaluation, randomized controlled trials (RCTs), etc. related to the research questions.
Retrieval strategy
The computer evidence retrieval was carried out according to the pyramid “6s” evidence model (6). The following databases were searched: UpToDate, British Medical Journal (BMJ) Best Practice, World Health Organization (WHO), Scottish Intercollegiate Guidelines Network (SIGN), Guidelines International Network (GIN), Australian Joanna Briggs Institute (JBI) Healthcare Database, Medlive, PubMed, Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang. The English search keywords were “after thymectomy/postoperation/after thymoma resection” AND “myasthenic crisis/myasthenia/myasthenia gravis”. The Chinese search keywords were “postoperative/post-thymectomy/post-thymoma resection” AND “myasthenic crisis/myasthenia/neuromuscular weakness/respiratory muscle weakness.” The search period was from the establishment of the databases to June 20, 2023. Taking PubMed as an example, the specific retrieval strategy is shown in Table 1.
Table 1
| #1 after thymectomy [All Fields] |
| #2 postoperation [All Fields] |
| #3 after thymoma resection [All Fields] |
| #4 #1 OR #2 OR #3 |
| #5 myasthenic crisis [All Fields] |
| #6 myasthenia [All Fields] |
| #7 myasthenia gravis [All Fields] |
| #8 #5 OR #6 OR #7 |
| #9 #4 AND #8 |
Literature inclusion and exclusion criteria
To be eligible for inclusion in this analysis, the articles had to meet the following inclusion criteria: (I) concern adult subjects who had undergone thymectomy; (II) concern research related to the evaluation, prevention, and management of postoperative MC; and (III) comprise one of the following types of literature: guidelines, expert consensus articles, clinical decisions, systematic reviews, RCTs, etc. Articles were excluded from the study if they met any of the following exclusion criteria: (I) comprised a conference paper, case report, review, or draft article; (II) failed in terms of quality; (III) included incomplete information, or the full text of the article could not be obtained; and (IV) were related to guidelines that had been updated.
Criteria for literature quality evaluation
The corresponding quality evaluation standards were selected based on the type of literature:
- The guidelines were evaluated using the Appraisal of Guidelines for Research and Evaluation (AGREE) II tool (7), which was updated in 2017 in the United Kingdom, and comprises a total of 23 items in six fields. Each item is evaluated on a scale of 1–7 (1= strongly disagree, 7= strongly agree). The standardized percentage of scores is calculated for each field, the field score is defined by the equation y = (x − b)/(a − b) × 100%, where y represents the field score, x represents the actual score, a represents the highest possible score, and b represents the lowest possible score; if the score in ≥5 fields is ≥60%, the guidelines are highly recommended (level A); if the score in ≥3 fields is ≥60%, the guidelines are recommended (level B); if the score in ≥3 fields is <30%, the guidelines are not recommended (level C).
- The expert consensus articles were evaluated using the expert consensus evaluation criteria of the Australian JBI Evidence-Based Healthcare Center [2016] (8), which comprises six evaluation items. Each evaluator independently selects one of the following responses for each item: “yes”, “no”, “unclear”, or “not applicable”.
- The clinical decisions were evaluated using the Critical Appraisal for Summaries of Evidence (CASE) (9), which includes 10 evaluation items. Each item has the following three options: “yes”, “partial yes”, and “no”.
- The systematic reviews were evaluated using the Assessment of Multiple Systematic Reviews (AMSTAR) evaluation criteria (10), which includes 11 items. Each evaluator independently selects one of the following responses for each item: “yes”, “no”, “unclear”, or “not adopted”.
- The RCTs were evaluated using the Cochrane bias risk assessment tool (11), which includes the following seven items: selection bias (random sequence generation or assignment hidden), implementation bias, measurement bias, follow-up bias, reporting bias, and other bias. Each evaluator independently makes a judgment of “low risk”, “high risk”, or “unclear” for each item.
Evidence level and recommendation level
The included evidence was then evaluated and graded using the Australian JBI Evidence-Based Healthcare Center Evidence Classification and Evidence Recommendation Level System (2014 edition) (12). According to the different types of research designs, the evidence level was divided into 1–5 levels. Based on the rigor and reliability of the research design, the recommendation level was divided into A-level recommendation (strong recommendation) and B-level recommendation (weak recommendation). The evidence recommendation was decided by the research team group. When there was a conflict between evidence from different sources, high-quality evidence was prioritized.
Literature quality evaluation process
The evidence team comprised four researchers who systematically studied evidence-based nursing. These researchers independently evaluated the quality of the literature, and extracted and summarized the evidence, fully considering the clinical significance and feasibility of that evidence.
Results
Literature search results
We first conducted a preliminary search and retrieved 1,148 relevant articles. We screened for duplicates by reading the titles and abstracts. Irrelevant and non-conforming articles were also excluded according to the exclusion criteria. Ultimately, 12 articles were included in the analysis, including three clinical guidelines, three expert consensus articles, three clinical decisions, two systematic reviews, and one RCT. The literature screening process is shown in Figure 1, and some general information about the included articles is shown in Table 2.
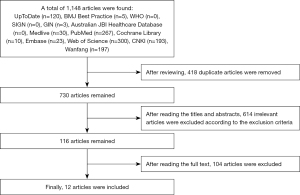
Table 2
| Authors | Publication year | Literature source | Evidence type | Literature theme |
|---|---|---|---|---|
| Narayanaswami et al. (13) | 2021 | BMJ Best Practice | Guidelines | International guidelines for the treatment of MG |
| Khan et al. (14) | 2023 | PubMed | Guidelines | Respiratory management in patients with neuromuscular weakness |
| Chinese Society of Immunology Neuroimmunology Branch (15) | 2021 | Wanfang | Guidelines | Chinese guidelines for the treatment and management of MG |
| Tan et al. (16) | 2022 | CNKI | Expert consensus article | Chinese clinical surgical treatment of MG |
| Evoli et al. (17) | 2019 | PubMed | Expert consensus article | Italian recommendations for the diagnosis and treatment of MG |
| Respiratory Therapy Group, Chinese Pathophysiological Critical Care Society (18) | 2020 | Medlive | Expert consensus article | Airway clearance in critically ill patients |
| Epstein et al. (19) | 2023 | UpToDate | Clinical decision | Management of respiratory muscle weakness caused by neuromuscular diseases |
| Bird et al. (20) | 2023 | UpToDate | Clinical decision | Emergency management and mechanical ventilation management of MC |
| Kveraga et al. (21) | 2023 | UpToDate | Clinical decision | Anesthesia management in patients with MG |
| Liu et al. (22) | 2020 | PubMed | Systematic review | Risk assessment of postoperative MC |
| Akaishi et al. (23) | 2019 | PubMed | Systematic review | Preoperative risk meta-analysis of postoperative MC |
| Chen et al. (24) | 2023 | PubMed | RCT | Preoperative respiratory muscle training combined with aerobic exercise can improve respiratory capacity and daily living activities after MG surgery |
BMJ, British Medical Journal; MG, myasthenia gravis; CNKI, China National Knowledge Infrastructure; MC, myasthenic crisis; RCT, randomized controlled trial.
Quality evaluation results of the included literature
Quality evaluation results of the guidelines
The three guidelines evaluated in this study were obtained from the BMJ Best Practice (13), PubMed (14), and Wanfang (15) databases. The guidelines were independently evaluated by the four reviewers. The standardized percentages of each field and the results of the two comprehensive evaluations are shown in Table 3. The study designs were complete, the overall quality was high, and these studies were approved for inclusion.
Table 3
| Authors | Standardized scores by field (%) | Number of fields ≥60% | Number of fields ≥30% | Overall quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Scope and purpose | Participants | Preciseness | Clarity | Applicability | Independence | ||||
| Narayanaswami et al. (13) | 88.9 | 76 | 76.8 | 64.6 | 48.7 | 95.8 | 5 | 6 | B |
| Khan et al. (14) | 93.1 | 91.7 | 85.1 | 71.9 | 79.2 | 72.9 | 6 | 6 | A |
| Chinese Society of Immunology Neuroimmunology Branch (15) | 88.9 | 68.8 | 51.2 | 67.7 | 55.6 | 62.5 | 4 | 6 | B |
Quality evaluation results of the expert consensus articles
The three expert consensus articles evaluated in this study were obtained from the CNKI (16), PubMed (17), and Medlive (18) databases. In relation to item 6, which states, “Is there any inconsistency between the viewpoints proposed and previous literature?”, the response for two articles (17,18) was “no”; however, the response for all the other articles was “yes”. The evaluation results in various fields are shown in Table 4; the study designs were complete, the overall quality was high, and these studies were approved for inclusion.
Table 4
| Authors | (I) Are the sources of the ideas clearly marked? | (II) Does the opinion come from influential experts in the field? | (III) Are the ideas presented centered on the interests of the relevant population? | (IV) Are the stated conclusions based on the analysis? Are ideas expressed logically? | (V) Does the article refer to other existing literature? | (VI) Is there any inconsistency between the proposed views and the previous literature? |
|---|---|---|---|---|---|---|
| Tan et al. (16) | Yes | Yes | Yes | Yes | Yes | Yes |
| Evoli et al. (17) | Yes | Yes | Yes | Yes | Yes | No |
| Respiratory Therapy Group, Chinese Pathophysiological Critical Care Society (18) | Yes | Yes | Yes | Yes | Yes | No |
Quality evaluation results of the clinical decisions
The three clinical decisions evaluated in this study were obtained from the UpToDate database (19-21), which is an evidence-based medicine database and an evidence resource at the top of the evidence pyramid with high-level, quality evidence. The evaluation results for all the items were “yes”. The study designs were complete, the overall quality was high, and these studies were approved for inclusion.
Quality evaluation results of the systematic reviews
The two systematic reviews evaluated in this study were obtained from the PubMed database (22,23). For one review (23), the response “unclear” was selected for item 11, which states: “Was a conflict of interest reported?”. However, for all the other reviews, the “yes” response was selected. The study designs were complete, the overall quality was high, and these studies were approved for inclusion.
Quality evaluation results of the RCT
One RCT was retrieved from the PubMed database. In the evaluation of the RCT (24), item 6, which concerned the “possibility of selective reporting of study results”, was evaluated as “unclear”; however, all the other items were evaluated as “low risk”. The study designs were complete, the overall quality was high, and this study was approved for inclusion.
Evidence summary and description
This study summarized evidence on the prevention and management of postoperative MC, and finally summarizes 39 best evidence.
Risk assessment and management
Risk assessment is the first step in the process of the nursing management of disease, and adequate preoperative preparation and assessment are crucial to the success of surgery and the prevention of crisis. The first and second pieces of evidence showed the risk factors for postoperative MC and the related drugs that can trigger the development of this complication. For controllable factors, intervention measures should be implemented to reduce the risk factors to a safe range; for uncontrollable factors, effective preventive measures should be implemented in a timely manner. The predictive identification of these risk factors could reduce the incidence of postoperative MC to a certain extent. Kanai et al. proposed a risk prediction model for postoperative MC (25), which has a sensitivity of 80% and a specificity of 87.8%, and can effectively predict the occurrence of postoperative MC.
At the same time, before and after surgery, multidisciplinary teams (MDTs) need to collaborate and engage in discussion for optimized management. MDTs, which comprise different professionals, such as surgeons, physicians, radiologists, and pathologists, evaluate a specific patient’s systemic condition, immune status, and cardiopulmonary function by consultation in a unified dedicated setting. Discussing the advantages and disadvantages of various treatment methods helps to formulate the best treatment plan and ensure the continuity of treatment (26).
The Myasthenia Gravis Foundation of America (MGFA) classifies mild, moderate, and severe myasthenia as MGFA types II, III, and IV, respectively, based on the results of the muscle strength test of each muscle group in the quantitative MG (QMG) score (27). This score objectively and carefully reflects the patient’s condition, and any changes and fluctuations before and after treatment. The QMG test enhances the objectivity and reliability of MGFA clinical classification, is conducive to prognostic analyses and therapeutic evaluation, is convenient for long-term follow-up evaluations and observations, and is suitable for clinical promotion and use.
Recommendation 1. Risk factors: a preoperative acetylcholine receptor antibody (AChR-Ab) level (>100 nmol/L); long-term use of glucocorticoids; WHO type B (B1, B2, and B3); a history of MC; ball symptoms; MG Osserman stages (IIb, III, and IV); pyridoxine use (>750 mg/day); lung function [vital capacity (VC) <2–2.9 L]; a disease duration <3 months; open thymectomy; incomplete resection of the thymoma; intraoperative blood loss >1,000 mL; postoperative inflammation (lung infection) (21-23). (Evidence level: 5c; recommendation: A).
Recommendation 2. MC inducing drugs: aminoglycoside antibiotics, antifungal drugs, cardiovascular drugs, anti-epileptic drugs, antipsychotics, anti-rheumatic drugs (13,15). (Evidence level: 5b; recommendation: B).
Recommendation 3. A multidisciplinary, collaborative MDT discussion should be conducted before surgery to evaluate the patient’s systemic condition, immune status, and cardiopulmonary function (13,17,21). (Evidence level: 5b; recommendation: A).
Recommendation 4. Follow-up treatment under MDT guidance after surgery should be provided. The clinical evaluation criteria of MGFA-Post Intervention Status (PIS) and the quantitative scale of clinical symptoms should be used. The QMG score should be used for evaluation and follow up (16,17). (Evidence level: 5b; recommendation: A).
Preoperative functional exercise
This study described specific methods for preoperative respiratory muscle training, sputum excretion promotion, and muscle training. Preoperative respiratory muscle training can improve the lung function reserve, help patients recover lung expansion after surgery, and guide patients to breathe correctly and cough, so that the postoperative habit becomes natural, patients can effectively cough and expectorate, and tension and anxiety can be relieved. Preoperative respiratory muscle training can also reduce the incidence of postoperative respiratory complications, and the occurrence of postoperative MC whereas preoperative exercise training can significantly improve the patient’s postoperative exercise ability (28).
Recommendation 5. Respiratory muscle training should be performed before surgery, including the use of lip breathing, abdominal breathing, and three-ball breathing trainers (the training amount is three balls per inhalation); training for 15 minutes, 3 times a day for 5 days, combined with aerobic exercise (up and down stairs) (16,24). (Evidence level: 5b; recommendation: A).
Recommendation 6. Chest physical therapy should be conducted (expectoration should be practiced 3 times a day for 15 minutes each time, for 5 days), forced exhalation expectoration should be practiced (3 times a day for 15 minutes each time, for 5 days), and smoking should cease for >2 weeks (16,24). (Evidence level: 1c; recommendation: A).
Recommendation 7. Limb muscle strength training using the United States Thera elastic band (red, moderate intensity), and aerobic exercise (10 times a day, three times each time, 1 minute rest) should be performed (24). (Evidence level: 1c; recommendation: B).
Recommendation 8. Patients taking anticholinesterase drugs (e.g., pyristostigmine and neostigmine) should continuing taking these drugs until the morning of surgery, and glucocorticoids should be adjusted to the lowest effective dose or discontinued completely (16,21). (Evidence level: 1c; recommendation: B).
Intraoperative
Thymectomy should be performed during a stable stage of the disease, when immunomodulatory drugs or glucocorticoids are at their lowest levels to minimize the likelihood of postoperative MC. As myasthenia symptoms are characterized as light in the morning and heavy in the evening, the surgery should be scheduled in the morning where possible to take advantage of the greater muscle strength typical of this day period (29). During the operation, the operative field should be exposed, and the thymus gland together with the perithymic fatty tissue contained within the anterior mediastinum should be removed as completely as possible, especially the adipose tissue at the base of the neck, bilateral pulmonary hilus, and behind the innominate vein, etc. During surgical maneuvering, care must be paid to prevent phrenic nerve injury (16,28).
The motor fibers of the phrenic nerve innervate the phrenic muscle, which is the main driving force of human breathing. After diaphragmatic nerve injury, ipsilateral hemidiaphragmatic paralysis may occur, and abdominal breathing may weaken or cease. In MG patients, diaphragmatic nerve injury can exacerbate breathing difficulties, and in severe cases, it can be life threatening. We recommend the continuous use of anticholinesterase drugs such as pyridostigmine up until and including the morning of the surgical day, while recognizing that these drugs may alter the patient’s response to both depolarizing and non-depolarizing neuromuscular blocking agent (NMBA). MG patients are often resistant to depolarizing NMBA but highly sensitive to non-depolarizing NMBA. Very low doses and residual non-depolarizing NMBA may cause respiratory distress after anesthesia recovery. Most surgical procedures in MG patients do not require the use of NMBA. If NMBA is necessary, we recommend the use of non-depolarizing NMBA such as rocuronium bromide and vecuronium bromide, which can be reversed with sugammadex rather than neostigmine. It has been reported that intravenous administration of sugammadex 2–4 mg/kg can reverse the blockade of mid-to-deep levels of rocuronium bromide and vecuronium bromide in MG patients within 4 minutes, the adequacy of blockade is determined by whether the train-of-four ratio (TOFR) is greater than 0.9 during quantitative neuromuscular monitoring (21). Inhalation anesthetics such as isoflurane and sevoflurane are favored due to their weak relaxation effect on skeletal muscles, low solubility in the blood, short duration of action, easy control of anesthesia depth, and the rapid recovery of nerve muscle transmission function after anesthesia.
Recommendation 9. Surgery should be scheduled as early in the day as possible, when the patient’s muscle strength is at its best (21). (Evidence level: 5b; recommendation: A).
Recommendation 10. The thymoma, all thymus and mediastinal fat should be completely removed. If the tumor is not completely removed, radiotherapy and chemotherapy should be performed after surgery (16). (Evidence level: 5b; recommendation: B).
Recommendation 11. Intraoperative phrenic nerve injury should be prevented (16). (Evidence level: 5b; recommendation: A).
Recommendation 12. Avoid NMBA as much as possible, if this is required, we recommend using rocuronium bromide or vecuronium bromide and then reversing with sugammadex (16,21). (Evidence level: 5b; recommendation: A).
Recommendation 13. Ultra-short-acting or short-acting sedatives, hypnotics, and anesthetics should be used to minimize respiratory depression upon recovery from anesthesia (21). (Evidence level: 5b; recommendation: A).
Postoperative
Precautions for extubation
The postoperative prevention strategy mainly focuses on early extubation, airway management, and respiratory secretion management. On the other hand, for patients who are deemed high-risk for MC after surgery, delaying extubation appropriately after surgery can effectively reduce both incidence of MC and risk of early re-intubation; a gastric tube can be placed before surgery to facilitate perioperative administration of pyridostigmine (19). The patient’s respiratory and muscle strength status should be closely monitored, and a clinical physician should determine the risk before removing the tube. Before removing the tube, the patient needs to undergo a spontaneous breathing trial (SBT) for a set period, usually 30 minutes to 2 hours. If after the removal of the tube, the VC drops to <15–20 mL/kg and the maximum inspiratory pressure (MIP) absolute value is <30 cmH2O, this indicates failure and another intubation is required.
The use of postoperative anticholinesterase drugs should be adjusted according to the patient’s condition after a discussion by the MDT, and the dose should be increased as appropriate. The daily dose should be less than 480 mg, and intravenous administration is not recommended.
Recommendation 14. The respiratory muscle strength of patients undergoing mechanical ventilation should be monitored every 4 hours, and the clinician should determine whether to extubate offline after evaluating the risk, and delay extubation if necessary (16,17,20). (Evidence level: 5b; recommendation: A).
Recommendation 15. Patients at risk of delayed extubation should have gastric tubes inserted before surgery to facilitate postoperative administration (17,20). (Evidence level: 5b; recommendation: A).
Recommendation 16. A small amount of ventilation support should be given prior to extubation, requiring the patient to tolerate experimental SBT for spontaneous breathing for a period, usually 30 minutes to 2 hours (19,20). (Evidence level: 5b; recommendation: B).
Recommendation 17. After successful extubation, the dose of cholinesterase inhibitor should be individually adjusted according to the fluctuation of the patient’s symptoms, and the dose should be increased as appropriate until it is within the safe dose range (<480 mg throughout the day), and intravenous administration is not recommended (15-17). (Evidence level: 5b; recommendation: A).
Recommendation 18. After extubation, all patients should be closely monitored for pulmonary VC and MIP value; a decrease in VC to <15–20 mL/kg, and an MIP absolute value <30 cmH2O indicate that extubation failure and the patient needs to be re-intubation (20). (Evidence level: 5b; recommendation: A).
Respiratory management
Respiratory muscle training is also necessary after surgery, and effective measures need to be taken to promote cough and sputum excretion, such as turning over and patting the back, nebulization inhalation, using tongue and pharyngeal respiration to increase lung capacity, administering multi-mode analgesia to reduce respiratory depression caused by pain, using artificial-assisted cough technology or mechanical-assisted cough technology to reduce cough ability, and administering appropriate treatments according to the quantity characteristics of the secretions. When there is a significant increase in respiratory secretions (i.e., an increase greater than 30 mL per day), a mucus regulator should be used.
In relation to viscous secretions using mucolytic agents, when the discharge of secretions is not smooth, mucus promoters and expectorants should be used (18). After surgery, due to the reduced ability to cough, secretions are not easily removed, and this, combined with the long-term use of hormones before surgery, can lead to metabolism disorders, suppressed immune function, an increase in the chance of infection, and induce the spread of lesions in the body. Actively seeking the causes of infections and the provision of timely treatments can help to reduce the incidence of postoperative MC.
An often-overlooked issue in perioperative care is the need for psychological support. Sudden emotional stress may increase muscle weakness, while chronic weakness may affect an individual’s mental and emotional health. A study has shown that patients are prone to anxiety, depression, and other adverse emotions due to the uncertainty of trauma and disease prognosis during the perioperative period (30). Therefore, attention should be paid to patients’ psychological state, and psychological interventions should be implemented to promote patients’ rehabilitation.
Recommendation 19. Respiratory function, coughing ability, and causes of sputum disturbance should be evaluated. Cough adequacy can be determined by regularly measuring the peak cough flow (PCF) rate; a PCF >160 L/min is required to effectively cough and clear respiratory secretions (18,19). (Evidence level: 5b; recommendation: A).
Recommendation 20. The patient should be instructed to use a breathing trainer (with the inspiratory pressure set to 30% of the maximum oral inspiratory pressure), and practice lip breathing, and abdominal breathing (24). (Evidence level: 5b; recommendation: A).
Recommendation 21. Airway care (e.g., turning the patient over regularly, patting back, and atomized inhalation) should be strengthened (15,19). (Evidence level: 1c; recommendation: A).
Recommendation 22. Glossopharyngeal breathing (GPB) should be used to increase lung capacity to promote coughing; inhaling should begin before exhalation completed; swallowing during GPB: swallow 50–60 mL of gas into the lungs, each “swallow” should take 0.5 seconds, repeat 10–12 times to achieve normal tidal volume or maximum tolerance of deep inspiratory volume (14). (Evidence level: 5b; recommendation: A).
Recommendation 23. For patients with reduced cough ability and respiratory muscle weakness, artificial assisted cough technology (e.g., the patient coughs spontaneously and forcefully, and the nurse gives abdominal impact at the same time) or mechanical cough assistance technology [non-invasive facemask mechanical insufflation-exsufflation (MIE)] should usually be administered once or twice a day, and the degree of cooperation and tolerance of the patient should be paid attention to during the operation to avoid barometric injury caused by man-machine confrontation (14,18,19). (Evidence level: 5b; recommendation: B).
Recommendation 24. Respiratory secretions should increase significantly (>30 mL/d) and mucus regulators should be used. Mucolytic agents should be used for viscous secretions; when the discharge of secretions is not smooth, mucus promoters and expectorants should be used (18). (Evidence level: 5b; recommendation: B).
Recommendation 25. If secretions are difficult to clear, high frequency chest wall oscillation, combined with cough, is recommended (20). (Evidence level: 5b; recommendation: A).
Recommendation 26. For postoperative hypersalivary patients who do not respond adequately to anticholinergic therapy, botulinum toxin (BT) therapy or salivary gland radiation therapy is recommended (14,19). (Evidence level: 5b; recommendation: B).
Recommendation 27. Care should be taken to correct electrolyte disorders and strengthen the monitoring of potassium, magnesium, phosphorus, etc. (19). (Evidence level: 5b; recommendation: B).
Recommendation 28. Recurrent infections are often the cause, and doctors should actively look for and address factors or medications that may trigger or worsen muscle weakness (18). (Evidence level: 5b; recommendation: A).
Identify pre-MC symptoms and emergency MC treatment
Research (31) indicates that 72.44% of patients with pre-MC will eventually develop MC. The early identification of pre-MC and effective treatment measures, such as the timely application of immunoglobulin, can rapidly improve the myasthenic state and significantly reduce the occurrence of MC. When MC has occurred, the emergency treatment is to open the airway, remove the retained carbon dioxide in the body, and perform ventilation therapy, intravenous immunoglobulin (IVIG) therapy, and plasma exchange (PE), after which medication should be administered according to each patient’s individual clinical condition. The respiratory rate, heart rate, dysphonia, and neck weakness must be closely monitored after surgery. Sometimes, general weakness and hypercapnia mask respiratory distress, all of which may indicate imminent respiratory failure. Any patient with a suspicious respiratory status of myasthenia should be admitted to intensive care unit for close observation. Suction devices, simple breathing apparatus, and emergency tracheal intubation materials should be made available at the bedside.
Recommendation 29. Difficulty breathing in the supine position can be described as “choking” or “drowning” (16,20). (Evidence level: 5b; recommendation: A).
Recommendation 30. Difficulty swallowing, accompanied by a weak cough, and difficulty in removing secretions (16,20,21). (Evidence level: 5a; recommendation: A).
Recommendation 31. Hypoarticulation, pausing for breath while speaking, weak breathing force, shallow and fast breathing caused by increased respiratory rate, use of auxiliary respiratory muscle breathing, and abnormal abdominal breathing (20,21). (Evidence level: 5a; recommendation: A).
Recommendation 32. No respiratory distress, but low baseline VC (<30 mL/kg ideal body weight) and elevated PCO2 and PaCO2 (20). (Evidence level: 5a; recommendation: A).
Recommendation 33. If expiratory failure (type I or type II) occurs, the following procedures should be implemented: timely intubation, positive pressure ventilation, and the use of auxiliary control modes of volume control ventilation and low-level positive end-expiratory pressure (PEEP). Additionally, sleep quality, leakage, blood oxygen, etc. should be continuously assessed, the best settings should be determined to avoid excessive ventilation, and anticholinesterase drugs should be suspended after intubation to avoid increased secretion (14,15,20). (Evidence level: 5a; recommendation: B).
Recommendation 34. Fast-acting treatments should be actively administered: Intravenous injection of human IVIG (400 mg/kg·d for 5 days) or PE (at a dose of 1.0 to 1.5 times the total plasma volume, 3 to 6 replacements are performed within 5 to 10 days, replacement fluid is healthy human plasma or albumin, accompanied by patients with caution). Respiratory function should be assessed, and arterial blood gas, the acid base balance state, and other indicators should be monitored to judge the type of MC (15,16). (Evidence level: 5b; recommendation: A).
Recommendation 35. Start immunosuppressive therapy (non-hormone immunosuppressants) such as azathioprine, tacrolimus, mycophenolate mofetil, etc. After the use of IVIG/PE, glucocorticoids can be used when the patient is stable (15,16). (Evidence level: 5b; recommendation: A).
Health education
Correct discharge health guidance is conducive to the prognosis and rehabilitation of patients. The lung function and chest computed tomography (CT) scans of patients need to be regularly reviewed to reduce any behavior that may aggravate MC. Patients need to rest and keep warm. Fatigue, the cold, and mood swings, etc., should be avoided. A doctor should be consulted before any drugs are taken, and patients with abnormal conditions need to attend hospital in time to avoid further deterioration. At present, a study has shown that Chinese medicine can regulate multiple links and aspects of specific and non-specific immune abnormalities in MG (32), and can exert multiple effects and superposition effects. After surgery, a treatment plan that integrates Chinese and Western medicine could be considered to gradually stimulate and mobilize the internal disease resistance activity of the human body and promote the human immune system to gradually return to the normal state. Disease recovery should be promoted, and the incidence of MC should be reduced.
Recommendation 36. Lung function tests should be performed every 3–6 months, and gas exchange parameters should be checked every 6 months (14,19). (Evidence level: 5b; recommendation: B).
Recommendation 37. Chest CT should be reviewed regularly, every 6 months for the first 2 years, and then once a year until 10 years after surgery (17). (Evidence level: 5b; recommendation: A).
Recommendation 38. Soapy water enema is prohibited; attention should be paid to ensure the patient rests and keeps warm; fatigue, cold, mood swings, etc., should be avoided (15,16). (Evidence level: 5b; recommendation: A).
Recommendation 39. Fatigue, drowsiness, a history of snoring, and episodes of asphyxia suggest a comprehensive polysomnography that can be used to evaluate whether to non-invasive positive pressure ventilation should be initiated (14,19). (Evidence level: 5b; recommendation: B).
Conclusions
Postoperative MC is a serious complication after thymectomy, and adequate understanding of postoperative MC characteristics and management strategies should be pursued. This study summarized the relevant evidence of postoperative MC prevention and management. It can provide strong guidance and is pertinent for the work of clinical medical staff.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-90/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-90/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 2009;8:475-90. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol 2019;18:259-68. [Crossref] [PubMed]
- Chen L, Xie W, Zheng D, et al. Early extubation after thymectomy is good for the patients with myasthenia gravis. Neurol Sci 2019;40:2125-32. [Crossref] [PubMed]
- Huang Y, Su L, Zhang Y, et al. Risk Factors for Postoperative Myasthenic Crisis After Thymectomy in Patients With Myasthenia Gravis. J Surg Res 2021;262:1-5. [Crossref] [PubMed]
- Neumann B, Angstwurm K, Mergenthaler P, et al. Myasthenic crisis demanding mechanical ventilation: A multicenter analysis of 250 cases. Neurology 2020;94:e299-313. [Crossref] [PubMed]
- Dicenso A, Bayley L, Haynes RB. Accessing pre-appraised evidence: fine-tuning the 5S model into a 6S model. Evid Based Nurs 2009;12:99-101. [Crossref] [PubMed]
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med 2010;51:421-4. [Crossref] [PubMed]
- The Joanna Briggs Institute. Available online: https://jbi.global/
- Foster MJ, Shurtz S. Making the Critical Appraisal for Summaries of Evidence (CASE) for evidence-based medicine (EBM): critical appraisal of summaries of evidence. J Med Libr Assoc 2013;101:192-8. [Crossref] [PubMed]
- Dosenovic S, Jelicic Kadic A, Vucic K, et al. Comparison of methodological quality rating of systematic reviews on neuropathic pain using AMSTAR and R-AMSTAR. BMC Med Res Methodol 2018;18:37. [Crossref] [PubMed]
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142. [Crossref] [PubMed]
- The University of Adelaide. The JBI Model of Evidence-based Healthcare. Available online: https://joannabriggs.org/
- Narayanaswami P, Sanders DB, Wolfe G, et al. International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology 2021;96:114-22. [Crossref] [PubMed]
- Khan A, Frazer-Green L, Amin R, et al. Respiratory Management of Patients With Neuromuscular Weakness: An American College of Chest Physicians Clinical Practice Guideline and Expert Panel Report. Chest 2023;164:394-413. [Crossref] [PubMed]
- Chinese Society of Immunology Neuroimmunology Branch. Chinese guidelines for the diagnosis and treatment of myasthenia gravis (2020 edition). Chin J Neuroimmunol Neurol 2021;28:1-12.
- Tan QY, Tao SL, Liu BD, et al. Chinese clinical expert consensus on surgical treatment of myasthenia gravis. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2022;29:529-41.
- Evoli A, Antonini G, Antozzi C, et al. Italian recommendations for the diagnosis and treatment of myasthenia gravis. Neurol Sci 2019;40:1111-24. [Crossref] [PubMed]
- Respiratory Therapy Group, Chinese Pathophysiological Critical Care Society. Expert consensus of airway clearance in critically ill patients. Chinese Journal of Critical Care & Intensive Care Medicine 2020;6:272-82. (Electronic Edition).
- Epstein SK. Respiratory muscle weakness due to neuromuscular disease: Management. 2023. Available online: https://www.uptodate.com
- Bird SJ, Levine JM. Myasthenic crisis. 2023. Available online: https://www.uptodate.com/
- Kveraga R, Pawlowski J. Anesthesia for the patient with myasthenia gravis. 2023. Available online: https://www.uptodate.com/
- Liu C, Liu P, Zhang XJ, et al. Assessment of the risks of a myasthenic crisis after thymectomy in patients with myasthenia gravis: a systematic review and meta-analysis of 25 studies. J Cardiothorac Surg 2020;15:270. [Crossref] [PubMed]
- Akaishi T, Motomura M, Shiraishi H, et al. Preoperative risks of post-operative myasthenic crisis (POMC): A meta-analysis. J Neurol Sci 2019;407:116530. [Crossref] [PubMed]
- Chen S, Li X, Wu Y, et al. Preoperative respiratory muscle training combined with aerobic exercise improves respiratory vital capacity and daily life activity following surgical treatment for myasthenia gravis. J Cardiothorac Surg 2023;18:160. [Crossref] [PubMed]
- Kanai T, Uzawa A, Sato Y, et al. A clinical predictive score for postoperative myasthenic crisis. Ann Neurol 2017;82:841-9. [Crossref] [PubMed]
- Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev 2016;42:56-72. [Crossref] [PubMed]
- Yang Y, Zhang M, Guo J, et al. Quality of life in 188 patients with myasthenia gravis in China. Int J Neurosci 2016;126:455-62. [Crossref] [PubMed]
- Rahbek MA, Mikkelsen EE, Overgaard K, et al. Exercise in myasthenia gravis: A feasibility study of aerobic and resistance training. Muscle Nerve 2017;56:700-9. [Crossref] [PubMed]
- Jamal BT, Herb K. Perioperative management of patients with myasthenia gravis: prevention, recognition, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:612-5. [Crossref] [PubMed]
- Jaruzel CB, Kelechi TJ. Relief from anxiety using complementary therapies in the perioperative period: A principle-based concept analysis. Complement Ther Clin Pract 2016;24:1-5. [Crossref] [PubMed]
- Ou CY, Ran H, Qiu L, et al. Correlation factors of 127 times pre-crisis state in patients with myasthenia gravis. Zhonghua Yi Xue Za Zhi 2017;97:2884-9. [PubMed]
- Xie R, Liu L, Wang R, et al. Traditional Chinese medicine for myasthenia gravis: Study protocol for a network meta-analysis. Medicine (Baltimore) 2020;99:e21294. [Crossref] [PubMed]


