
Development and validation of a nomogram for preoperative prediction of ipsilateral cervical central lymph node metastasis in papillary thyroid cancer: a population-based study
Highlight box
Key findings
• We integrated multiple factors to establish a nomogram-based preoperative prediction model of ipsilateral cervical central lymph node metastasis (CLNM) in papillary thyroid cancer (PTC).
What is known and what is new?
• The role of prophylactic central neck dissection in papillary thyroid cancer patients is controversial and it is still a matter of the debate.
• A non-invasive, and accurate nomogram prediction model for ipsilateral cervical CLNM of PTC was established. It can help physicians identify patients with a high risk of ipsilateral cervical CLNM of PTC preoperative for individualized treatment.
What is the implication, and what should change now?
• The preoperative prediction of the risk of central lymph node metastases and the decision on the necessity and the extent of prophylactic lymphadenectomy is crucial.
IntroductionOther Section
Thyroid cancer, as one of the most common malignant tumors, is rapidly increasing in incidence (1), and papillary thyroid cancer (PTC) is the most prevalent subtype of thyroid cancer (2). Lymph node metastasis (LNM) is the main route of PTC metastasis with an incidence of 17–36% (3), and especially cervical central lymph node metastasis (CLNM) is the most common. Understanding the extent and location of LNM is crucial for developing treatment plans. Studies have confirmed that PTC patients with CLNM have a lower survival rate and a higher risk of recurrence (4,5). Therefore, it has been suggested that prophylactic central lymph node dissection can identify lymph node lesions and potentially reduce the risk of disease recurrence (6). However, it has also been suggested that prophylactic central lymph node dissection does not appear to improve the prognosis of patients with low-risk PTC (7), but rather increases the incidence of surgical complications and the risk of long-term postoperative hormone replacement therapy, resulting in overtreatment. Compared to bilateral or distant metastasis, patients with ipsilateral LNM generally have a better prognosis. For patients with ipsilateral cervical CLNM, it may be sufficient to perform ipsilateral lymph node dissection, avoiding intervention on the contralateral side of the neck to reduce surgical risks and complications. Therefore, assessing the risk of ipsilateral cervical CLNM in patients with PTC and treating them with precise surgical procedures have become a hot topic of current research. Currently, clinical suspicion of CLNM requires invasive tests such as fine needle aspiration biopsy of the thyroid and lymph nodes or expensive genetic diagnosis to aid in the evaluation. There is a lack of noninvasive, effective, and convenient assessment tools for the preoperative recognition of CLNM.
Nomogram prediction models are widely used in clinical cohort studies because of their high accuracy, efficiency, and stability. Although studies have identified independent risk factors for constructing predictive models for CLNM in PTC, these studies were more often based on ultrasound (8,9) or computed tomography (CT) images (10,11), with insufficient clinical indicators and thus yielding inconsistent results. Some studies (12,13) used a public database that does not necessarily apply to Asian populations due to ethnicity.
Our study was based on the Chinese population from a practical clinical application scenario, integrating multidimensional factors such as patient demographic characteristics, medical history, preoperative biochemical examination, bone mineral density examination, ultrasound image characteristics, and pathology, to establish and validate a prediction model for early ipsilateral cervical CLNM in PTC based on nomogram. The model is noninvasive, simple, and effective, and can assist physicians in better performing preoperative identification of patients with a high risk of ipsilateral cervical CLNM in PTC and to develop individualized and highly accurate treatment plans. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-478/rc).
MethodsOther Section
Participants
This study is a retrospective cohort study. This study included 609 patients who underwent thyroid lobectomy and ipsilateral central compartment lymph node dissection at Peking University International Hospital from February 2018 to February 2021. Inclusion criteria: (I) age >18 years; (II) pathological diagnosis of PTC; (III) with complete demographic characteristics, biochemical indices, preoperative ultrasound information, and postoperative pathological data. Exclusion criteria: (I) patients with recurrent thyroid cancer; (II) combined with other malignant tumors; (III) preoperative thyroid nodules which had received adjuvant treatment such as oncological radiotherapy and thyroid nodule ablation.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was retrospective and approved by the Ethics Committee of Peking University International Hospital (No. 2022-KY-0040-01). Because this study used retrospective data and involved no direct contact with study participants, the requirement for informed consent was waived by the Ethics Committee.
Procedures
The following data were collected through the hospital case system: demographic characteristics [sex, age, weight, height, systolic blood pressure (SBP), diastolic blood pressure (DBP)], medical history [Hashimoto’s thyroiditis (HT)], biochemical examinations (thyroid function, thyroid antibodies, liver and kidney function, calcium and phosphorus, blood glucose, lipids), bone density examination.
This study retrospectively retrieved and assessed the preoperative thyroid ultrasound images of 609 patients pathologically confirmed with papillary thyroid carcinoma. These images were reevaluated by experienced radiologists (with over 10 years of experience in thyroid imaging). The assessment covered the number of thyroid nodules, size, echo, nature, boundary, shape, location, blood flow signals, microcalcification of the cancerous nodules, enlarged lymph nodes (ELN) in the central neck, and extrathyroidal extension (ETE) in the images. The method of partitioning the cervical lymph nodes followed the American Joint Committee on Cancer (AJCC) seven-part division of the cervical lymph nodes (14). We defined a lymph node with a short axis >0.8 cm as an ELN (15).
All enrolled PTC patients (n=609) were divided into two sets, using a random sampling method in a 7:3 ratio, the modeling set (n=426), and the validation set (n=183). Each data set was divided into two groups based on ipsilateral cervical central compartment lymph node pathology: the CLNM group and the non-CLNM group (as shown in Figure 1).
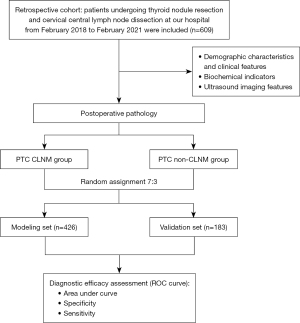
Statistical analysis
Data were analyzed using SPSS 26.0 software. Normally distributed data were expressed as mean ± standard deviation (
ResultsOther Section
Clinical characteristics of subjects in the modeling set (Table 1)
Table 1
| Index | CLNM group | Non-CLNM group | t/U/χ2 | P |
|---|---|---|---|---|
| Patients | 109 (25.6) | 317 (74.4) | ||
| Gender (male) | 27 (24.8) | 65 (20.5) | 0.872 | 0.35 |
| Age (years) | 40.00 (33.00, 52.50) | 47.00 (37.00, 58.00) | −3.420 | <0.001* |
| BMI (kg/m2) | 24.2±2.9 | 24.2±3.5 | −3.910 | 0.96 |
| Osteoporosis (yes) | 8 (7.3) | 5 (1.6) | 9.103 | 0.003* |
| HT (yes) | 36 (33.0) | 43 (13.6) | 20.34 | <0.001* |
| SBP (mmHg) | 125.00 (120.00, 135.00) | 126.00 (120.00, 136.00) | −1.260 | 0.21 |
| DBP (mmHg) | 78.00 (70.00, 80.00) | 75.00 (70.00, 80.00) | −0.887 | 0.38 |
| TSH (µIU/mL) | 2.25 (1.34, 2.93) | 1.81 (1.14, 2.57) | −1.933 | 0.04* |
| FT4 (pmol/L) | 15.50 (14.30, 16.90) | 16.06 (14.70, 17.60) | −1.664 | 0.10 |
| FT3 (pmol/L) | 4.71 (4.20, 5.20) | 4.70 (4.20, 4.90) | −1.608 | 0.11 |
| TRAb positive | 0 | 4 (1.3) | 1.388 | 0.24 |
| TgAb positive | 46 (42.2) | 128 (40.4) | 0.112 | 0.74 |
| TPOAb positive | 22 (20.2) | 36 (11.4) | 5.373 | 0.02* |
| FBG (mmol/L) | 4.40 (3.70, 4.80) | 4.30 (3.70, 4.67) | −0.42 | 0.67 |
| TC (mmol/L) | 4.59 (3.86, 4.65) | 4.59 (3.98, 5.11) | −0.45 | 0.65 |
| TG (mmol/L) | 2.04 (0.88, 2.14) | 2.02 (0.96, 2.13) | −0.734 | 0.46 |
| HDL-C (mmol/L) | 1.18 (1.00, 1.32) | 1.19 (0.88, 1.35) | −0.931 | 0.35 |
| LDL-C (mmol/L) | 2.66 (2.16, 2.72) | 2.65 (2.14, 3.18) | −1.573 | 0.12 |
| ALT (U/L) | 18.00 (12.00, 25.50) | 18.00 (12.00, 22.00) | −0.107 | 0.92 |
| AST (U/L) | 20.00 (15.00, 24.00) | 19.00 (16.00, 22.00) | −0.194 | 0.85 |
| UA (µmol/L) | 314.23 (246.00, 351.50) | 313.32 (257.50, 357.00) | −0.219 | 0.83 |
| eGFR (mL/min×1.73 m2) | 100.18 (98.76, 113.07) | 105.20 (100.78, 107.91) | −2.138 | 0.03* |
| Ca (mmol/L) | 2.33 (2.25, 2.35) | 2.31 (2.20, 2.35) | −0.955 | 0.34 |
| P (mmol/L) | 1.21 (1.20, 1.25) | 1.22 (1.17, 1.24) | −0.532 | 0.60 |
Categorical data are expressed as n (%). Normally distributed data are expressed as mean ± standard deviation and non-normally distributed data were expressed as median (interquartile range). *, P<0.05 vs. CLNM group. CLNM, central lymph node metastasis; BMI, body mass index; HT, Hashimoto’s thyroiditis; SBP, systolic blood pressure; DBP, diastolic blood pressure; TSH, thyroid stimulating hormone; FT4, free tetraiodothyronine; FT3, free triiodothyronine; TRAb, thyrotropic receptor antibody; TgAb, anti-thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; FBG, fasting blood glucose; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate transaminase; UA, uric acid; eGFR, estimated glomerular filtration rate; Ca, calcium; P, phosphorus.
Of the 426 patients with PTC included in the study, a total of 109 patients (25.6%) with pathologically confirmed CLNM, 24.8% of whom were males, were included in the CLNM group. A total of 317 patients (74.4%) without CLNM, 20.5% of whom were males, were included in the non-CLNM group.
The age of subjects in the CLNM group was significantly lower than that in the non-CLNM group (P<0.001). The proportion of subjects with osteoporosis (7.3% vs. 1.6%, P=0.003) and complicated by HT (33.0% vs. 13.6%, P<0.001) was significantly higher in the CLNM group compared with the non-CLNM group. The thyroid stimulating hormone (TSH) level was significantly higher in the CLNM group than in the non-CLNM group (P=0.04). The proportion of thyroid peroxidase antibody (TPOAb) positive was higher in the CLNM group than in the non-CLNM group (20.2% vs. 11.4%, P=0.02). The estimated glomerular filtration rate (eGFR) was lower in the CLNM group than in the non-CLNM group (P=0.03). There was no significant difference in body mass index (BMI), history of alcohol consumption, SBP, free tetraiodothyronine (FT4), free triiodothyronine (FT3), DBP, anti-thyroglobulin antibody (TgAb), thyrotropic receptor antibodies (TRAb), fasting blood glucose (FBG), lipids, alanine aminotransferase (ALT), aspartate transaminase (AST), uric acid (UA), calcium (Ca), and phosphorus (P) levels between the two groups.
Ultrasonic characteristics of subjects in the modeling set (Table 2)
Table 2
| Index | CLNM group (n=109) | Non-CLNM group (n=317) | χ2 | P |
|---|---|---|---|---|
| Multifocal (yes) | 74 (67.9) | 191 (60.3) | 2.012 | 0.15 |
| Features of cancerous nodule | ||||
| Size (>1 cm) | 78 (71.6) | 175 (55.2) | 8.995 | 0.003* |
| Echo | 6.344 | 0.18 | ||
| Unclear | 4 (3.7) | 14 (4.4) | ||
| Low | 85 (78.0) | 260 (82.0) | ||
| Media | 7 (6.4) | 21 (6.6) | ||
| High | 8 (7.3) | 7 (2.2) | ||
| Mixed | 5 (4.6) | 15 (4.7) | ||
| Nature | 1.218 | 0.75 | ||
| Unclear | 1 (0.9) | 7 (2.2) | ||
| Capsular | 3 (2.8) | 7 (2.2) | ||
| Solidity | 92 (84.4) | 258 (81.4) | ||
| Cystic solidity | 13 (11.9) | 45 (14.2) | ||
| Boundary (blurred) | 90 (82.6) | 225 (71.0) | 5.656 | 0.02* |
| Shape (irregular) | 94 (86.2) | 237 (74.8) | 6.164 | 0.01* |
| Location (vertical) | 82 (75.2) | 258 (81.4) | 1.909 | 0.17 |
| BFS (yes) | 76 (69.7) | 203 (64.0) | 1.161 | 0.28 |
| Microcalcification (yes) | 61 (56.0) | 117 (36.9) | 12.107 | 0.001* |
| ELN (yes) | 47 (43.1) | 47 (14.8) | 37.756 | <0.001* |
| ETE (yes) | 64 (58.7) | 92 (29.0) | 30.813 | <0.001* |
Data are expressed as n (%). *, P<0.05 vs. CLNM group. CLNM, central lymph node metastasis; BFS, blood flow signals; ELN, enlarged lymph nodes; ETE, extrathyroidal extension.
Ultrasound imaging showed a higher proportion of nodules >1 cm (71.6% vs. 55.2%, P=0.003), blurred borders (82.6% vs. 71.0%, P=0.02), irregular shape (86.2% vs. 74.8%, P=0.01), microcalcification (56.0% vs. 36.9%, P=0.001), ELN in the central neck (43.1% vs. 14.8%, P<0.001) and ETE (58.7% vs. 29.0%, P<0.001) in the CLNM group compared to the non-CLNM group.
Logistic regression analysis of risk factors of ipsilateral cervical CLNM in the modeling set
In univariate analysis (Tables 1,2), the following factors were associated with ipsilateral cervical CLNM: age, history of osteoporosis, complicated by HT, TSH level, TPOAb, eGFR level, cancerous nodule size, cancerous nodule boundary, cancerous nodule shape, microcalcification, ELN in the central neck and ETE.
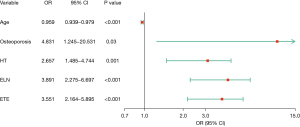
Then, we performed binary logistic regression analysis on the above risk factors, and the results showed that age, history of osteoporosis, complicated by HT, ELN in the central neck and ETE were risk factors for ipsilateral cervical CLNM (as shown in Figure 2). The younger the age, the higher the risk of ipsilateral cervical CLNM (95% CI: 0.939–0.979, P<0.001). The risk of ipsilateral cervical CLNM was 4.831 times higher in patients with osteoporosis (95% CI: 1.245–20.531, P=0.03). Patients with PTC who have HT experience a 2.657-fold increase in the risk of ipsilateral cervical CLNM (95% CI: 1.485–4.744, P=0.001). The risk of ipsilateral cervical CLNM was 3.891 times (95% CI: 2.275–6.697, P<0.001) higher in patients with ELN in the central neck than in patients with normal lymph nodes. Patients with PTC who exhibit ETE have a 3.551-fold increased risk of ipsilateral central CLNM (95% CI: 2.164–5.895, P<0.001).
Nomogram and evaluation of prediction model of ipsilateral cervical CLNM
Based on the independent risk factors for ipsilateral cervical CLNM, a nomogram of the ipsilateral cervical CLNM risk prediction model for PTC patients was developed (as shown in Figure 3), and the total ipsilateral cervical CLNM score for PTC patients was calculated based on the scores corresponding to each predictor to estimate the probability of ipsilateral cervical CLNM. The cutoff value for the nomogram score was obtained from the maximum Youden index, and this value was used to differentiate whether the patient was at high risk for ipsilateral cervical CLNM.

We evaluated the ROC curves for each independent risk factor predicting ipsilateral cervical CLNM separately (as shown in Figure 4A), with an AUC of 0.610 (95% CI: 0.547–0.672) for age, 0.529 (95% CI: 0.503–0.554) for the history of osteoporosis, 0.597 (95% CI: 0.549–0.646) for complicated by HT, 0.641 (95% CI: 0.591–0.692) for ELN in the central neck and 0.648 (95% CI: 0.596–0.701) for ETE.
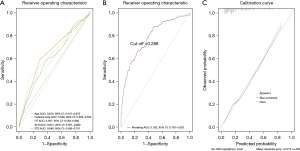
The AUC of the ipsilateral cervical CLNM risk prediction model in the modeling set population was 0.782 (95% CI: 0.730–0.833), with a sensitivity and specificity of 0.761 and 0.763, respectively (as shown in Figure 4B), which had a good diagnostic performance. The model presented better discrimination than any of the independent risk factors. The calibration curve for the risk of ipsilateral cervical CLNM showed good agreement between the predicted and actual observations in the nomogram (as shown in Figure 4C). The diagnostic cutoff value of this nomogram prediction model for distinguishing a PTC population at high risk of CLNM was 0.288.
The AUC of the ipsilateral cervical CLNM risk prediction model in the internal validation set population was 0.753 (95% CI: 0.648–0.858), and the sensitivity and specificity of the validation model were 0.661 and 0.863, respectively, with good discriminatory power (as shown in Figure 5A). The calibration curves of the validation set also showed good agreement (as shown in Figure 5B).

DiscussionOther Section
In this study, a variety of factors such as patient demographic characteristics, medical history, preoperative biochemical examinations, bone mineral density examinations, and imaging features were fully integrated for more accurate modeling to assess the risk of ipsilateral cervical CLNM in PTC. A total of five independent risk factors were identified, and patients with younger age, history of osteoporosis, complicated by HT, ELN in the central neck and ETE had a greater risk of CLNM. ETE (AUC 0.648) had the highest predictive value for ipsilateral cervical CLNM of PTC, followed by ELN in the central neck (AUC 0.641), complicated by HT (AUC 0.597), age (AUC 0.610) and history of osteoporosis (AUC 0.529).
ETE refers to the phenomenon where thyroid cancer cells spread beyond the thyroid capsule and invade surrounding tissues. Numerous studies (16-18) have shown that ETE is an independent risk factor for LNM in PTC. Consistent with previous research findings, we observed that PTC patients with ETE face a higher risk of ipsilateral cervical CLNM. ETE can increase the likelihood of tumor cells spreading through the lymphatic system, thereby elevating the risk of LNM. Consequently, for PTC patients with ETE, more aggressive treatment strategies may need to be considered, such as extensive cervical lymph node dissection and adjuvant therapy post-surgery.
Previous studies have identified ELN (19,20) as a risk factor for CLNM, and we obtained the same results. Tumor cells can spread from the primary thyroid tumor site to the central lymph nodes through the lymphatic system, and ELN is often considered a direct clinical sign of LNM in PTC. However, not all ELN are attributable to metastasis from PTC; ELN can also be due to infections, inflammation, or other types of cancer. In cases of micrometastases, patients with LNM may not exhibit significant ELN. Therefore, the specificity of ELN in the central neck for predicting CLNM in PTC is not high and it needs to be combined with other predictors.
HT is an autoimmune thyroid disease characterized by chronic lymphocytic thyroiditis. In recent years, researchers have extensively explored the relationship between HT and PTC, particularly the potential impact of HT on LNM in PTC, yet the findings have been inconsistent. Some studies have found a significant effect of HT on the risk of LNM in PTC (21-23). Our findings are in line with this, indicating an increased risk of ipsilateral cervical CLNM among PTC patients with concurrent HT, although the specific mechanisms remain unclear. Other studies have indicated that patients with HT present with lower aggressiveness at onset and better prognosis in PTC (24,25), suggesting that the chronic inflammatory environment induced by HT may limit cancer cell invasion and metastasis through enhanced local immune surveillance. A study found (26) that in PTC patients with the BRAF wild-type, the presence of HT appears to offer a more pronounced protective factor against the risk of disease recurrence, a protective effect that seems less significant in patients with BRAF mutations. Unfortunately, our study lacks genetic data, preventing stratified analysis. Therefore, future prospective studies are needed to further explore these relationships.
Consistent with previous studies (16,22,27,28), our study found that youth was a risk factor for CLNM in patients with PTC. In addition, it has been confirmed (29) that the survival rate of PTC patients combined with CLNM was significantly lower in those under 45 years of age, and the diagnostic threshold for age in our study was 42 years. This might be because young individuals have higher basal metabolic rates and are more likely to have things like genetic abnormalities (30). In older patients, although the risk of LNM may be lower, once metastasis occurs, the prognosis could be poorer. Therefore, age and other relevant factors should be considered comprehensively when formulating treatment plans and prognostic assessments.
In addition, the present study adequately evaluated preoperative clinical indicators and found that a history of osteoporosis was an independent risk factor for CLNM in patients with PTC, which has rarely been mentioned in previous studies. This may be because most of the indicators in previous studies were measured postoperatively, and patients were not evaluated for osteoporosis preoperatively. However, due to study sample size limitations, subsequent larger and more case-cohort studies are needed to assess the relationship and application of skeletal health impairment and CLNM in PTC. PTC is usually inert, however, 4% of patients with PTC present with distant lesions at the time of diagnosis (31). Bone is a common site of distant tumor localization and bone metastases have a potential negative impact on the bone microenvironment and bone strength (32). Therefore, a preoperative assessment of bone mineral density should be necessary for patients with PTC.
Furthermore, previous studies (18,22,33) have identified several risk factors for cervical CLNM in PTC, such as microcalcifications, nodule size, and multifocality. Microcalcifications are an important ultrasonographic feature in the diagnosis of PTC. Moreover, microcalcifications may be associated with aggressive tumor features, such as the risk of LNM. However, it is important to note that not all PTC patients with microcalcifications will develop LNM. Therefore, the presence of microcalcifications should be considered in conjunction with other clinical and radiological features to assess the patient’s risk comprehensively. Typically, larger thyroid nodules are associated with a higher risk of LNM. Larger tumors may imply a higher tumor burden, thereby increasing the chances of LNM. However, this relationship is not absolute, as even micro-PTCs smaller than 1 cm can undergo LNM. In this study, we found that even for thyroid nodules <1 cm, 17.9% (31/173) of patients still developed CLNM. While nodule size is an important indicator for assessing the risk of LNM, this relationship is influenced by various factors, including the biological characteristics of the tumor and other clinical features. Therefore, when assessing the risk of LNM in thyroid cancer patients, nodule size should be considered in conjunction with other diagnostic indicators. Multifocality of nodules may increase the risk of LNM, as multifocal PTC is often associated with a higher tumor burden, possibly making patients more susceptible to aggressive cancer behaviors, such as LNM. Additionally, multifocality may reflect underlying genetic predispositions or environmental factors, promoting carcinogenesis in multiple areas of the thyroid. However, this study did not find an association between microcalcifications, nodule size, and multifocality with CLNM in patients with PTC. This may be influenced by various factors, including a small sample size, the study being of a retrospective cohort design, differences in population characteristics, data analysis methods, and differences in the study setting.
The nomogram is a graphical computational ruler that has been widely used in medicine in recent years by combining important parameters of regression analysis. The prediction model built on the nomogram in this study integrated multiple risk factors with an AUC of 0.782 (95% CI: 0.730–0.833), which was higher than any of the independent risk factors, and had a higher discriminatory and validation ability to identify the risk of PTC combined with neck CLNM more effectively. The ROC best cut-off values showed sensitivity and specificity of 76.1% and 76.3%, respectively. In the external validation group, the nomogram also performed well with an AUC of 0.753 (95% CI: 0.648–0.858). The present nomogram model can help clinicians screen the high-risk group of ipsilateral cervical CLNM in this group of patients to help develop individualized treatment strategies.
However, this study does have some limitations. The thyroid ultrasound examinations were not standardized across operators, potentially introducing bias. Owing to the retrospective and non-randomized nature of this study, many significant data were not included in the analysis. For instance, all patients underwent only ipsilateral central neck lymph node dissection, leaving the status of the contralateral central lymph nodes unknown. This study was unable to specifically identify which nodules in multifocal presentations were malignant, with scenarios potentially involving either a single malignant nodule or multiple malignant nodules. Data on patients’ previous thyroid disease history, such as the presence of Graves’ disease, were also incomplete. Enhanced cervical CT and fine-needle aspiration were not routinely used in this study and were limited to a very small number of patients, restricting the possibility of in-depth analysis. Important information, such as genetic data, was also missing from our study data. Additionally, this study had a small sample size and lacked external validation. Therefore, large-scale prospective studies are needed to further analyze these issues and validate the clinical value of the model.
ConclusionsOther Section
In conclusion, age, history of osteoporosis, complicated by HT, ELN in the central neck, and ETE were found to be risk factors for ipsilateral cervical CLNM in PTC in this study. A nomogram prediction model was created by combining these five independent risk factors to more accurately predict the likelihood of ipsilateral cervical CLNM. This model has good diagnostic efficacy, which can assist doctors in better preoperative identification of high-risk groups of PTC ipsilateral cervical CLNM.
AcknowledgmentsOther Section
The authors thank the participants for participating in the study and the medical staff for collecting information. The authors would also like to thank the doctors of the Ultrasound Department at Peking University International Hospital for their assistance with the ultrasound imaging evaluation.
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-478/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-478/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-478/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-478/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was retrospective and approved by the Ethics Committee of Peking University International Hospital (No. 2022-KY-0040-01). Because this study used retrospective data and involved no direct contact with study participants, the requirement for informed consent was waived by the Ethics Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Pizzato M, Li M, Vignat J, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol 2022;10:264-72. [Crossref] [PubMed]
- Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol 2020;16:17-29. [Crossref] [PubMed]
- Weng HY, Yan T, Qiu WW, et al. The Prognosis of Skip Metastasis in Papillary Thyroid Microcarcinoma Is Better Than That of Continuous Metastasis. J Clin Endocrinol Metab 2022;107:1589-98. [Crossref] [PubMed]
- Kuczma P, Demarchi MS, Leboulleux S, et al. Central node dissection in papillary thyroid carcinoma in the era of near-infrared fluorescence. Front Endocrinol (Lausanne) 2023;14:1110489. [Crossref] [PubMed]
- Wu Z, Han L, Li W, et al. Which is preferred for initial treatment of papillary thyroid cancer, total thyroidectomy or lobotomy? Cancer Med 2021;10:1614-22. [Crossref] [PubMed]
- Pastorčić Grgić M, Stubljar B, Perše P, et al. Total Thyroidectomy with Central Node Dissection is a Valuable Option in Papillary Thyroid Cancer Treatment. Acta Clin Croat 2020;59:102-7. [Crossref] [PubMed]
- Ahn JH, Kwak JH, Yoon SG, et al. A prospective randomized controlled trial to assess the efficacy and safety of prophylactic central compartment lymph node dissection in papillary thyroid carcinoma. Surgery 2022;171:182-9. [Crossref] [PubMed]
- Chen Q, Liu Y, Liu J, et al. Development and validation of a dynamic nomogram based on conventional ultrasound and contrast-enhanced ultrasound for stratifying the risk of central lymph node metastasis in papillary thyroid carcinoma preoperatively. Front Endocrinol (Lausanne) 2023;14:1186381. [Crossref] [PubMed]
- Sun Y, Sun W, Xiang J, et al. Corrigendum: Nomogram for predicting central lymph node metastasis in T1-T2 papillary thyroid cancer with no lateral lymph node metastasis. Front Endocrinol (Lausanne) 2023;14:1148920. [Crossref] [PubMed]
- Tong Y, Li J, Huang Y, et al. Ultrasound-Based Radiomic Nomogram for Predicting Lateral Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Acad Radiol 2021;28:1675-84. [Crossref] [PubMed]
- Zhao W, Shen S, Ke T, et al. Clinical value of dual-energy CT for predicting occult metastasis in central neck lymph nodes of papillary thyroid carcinoma. Eur Radiol 2024;34:16-25. [Crossref] [PubMed]
- Lu Y, Qian K, Fei M, et al. A prognostic nomogram for papillary thyroid cancer lymph node metastasis based on immune score. Front Endocrinol (Lausanne) 2022;13:993856. [Crossref] [PubMed]
- Feng Y, Min Y, Chen H, et al. Construction and validation of a nomogram for predicting cervical lymph node metastasis in classic papillary thyroid carcinoma. J Endocrinol Invest 2021;44:2203-11. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Prativadi R, Dahiya N, Kamaya A, et al. Chapter 5 Ultrasound Characteristics of Benign vs Malignant Cervical Lymph Nodes. Semin Ultrasound CT MR 2017;38:506-15. [Crossref] [PubMed]
- Mao J, Zhang Q, Zhang H, et al. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:265. [Crossref] [PubMed]
- Song RY, Kim HS, Kang KH. Minimal extrathyroidal extension is associated with lymph node metastasis in single papillary thyroid microcarcinoma: a retrospective analysis of 814 patients. World J Surg Oncol 2022;20:170. [Crossref] [PubMed]
- Huang Y, Yin Y, Zhou W. Risk Factors for Central and Lateral Lymph Node Metastases in Patients With Papillary Thyroid Micro-Carcinoma: Retrospective Analysis on 484 Cases. Front Endocrinol (Lausanne) 2021;12:640565. [Crossref] [PubMed]
- Deng Y, Zhang J, Wang J, et al. Risk factors and prediction models of lymph node metastasis in papillary thyroid carcinoma based on clinical and imaging characteristics. Postgrad Med 2023;135:121-7. [Crossref] [PubMed]
- Wei X, Wang X, Xiong J, et al. Risk and Prognostic Factors for BRAF(V600E) Mutations in Papillary Thyroid Carcinoma. Biomed Res Int 2022;2022:9959649. [Crossref] [PubMed]
- Wang D, Hu J, Deng C, et al. Predictive nomogram for central lymph node metastasis in papillary thyroid microcarcinoma based on pathological and ultrasound features. Front Endocrinol (Lausanne) 2023;14:1108125. [Crossref] [PubMed]
- Zeng B, Min Y, Feng Y, et al. Hashimoto's Thyroiditis Is Associated With Central Lymph Node Metastasis in Classical Papillary Thyroid Cancer: Analysis from a High-Volume Single-Center Experience. Front Endocrinol (Lausanne) 2022;13:868606. [Crossref] [PubMed]
- Marongiu A, Nuvoli S, De Vito A, et al. Hashimoto's Thyroiditis and Papillary Thyroid Carcinoma: A Follow-Up Study in Patients with Absence of Aggressive Risk Factors at the Surgery of the Primary Tumor. Diagnostics (Basel) 2023;13:3068. [Crossref] [PubMed]
- Huang H, Xu S, Ni S, et al. Hashimoto's thyroiditis is negatively associated with lymph node metastasis in PTMC. J Cancer Res Clin Oncol 2023;149:15525-33. [Crossref] [PubMed]
- Xu S, Huang H, Qian J, et al. Prevalence of Hashimoto Thyroiditis in Adults With Papillary Thyroid Cancer and Its Association With Cancer Recurrence and Outcomes. JAMA Netw Open 2021;4:e2118526. [Crossref] [PubMed]
- Issa PP, Omar M, Buti Y, et al. Hashimoto's Thyroiditis: A Protective Factor against Recurrence in BRAF-Wild Type Differentiated Thyroid Carcinoma. Cancers (Basel) 2023;15:2371. [Crossref] [PubMed]
- Kim DH, Kim SW, Hwang SH. Predictive Value of Delphian Lymph Node Metastasis in the Thyroid Cancer. Laryngoscope 2021;131:1990-6. [Crossref] [PubMed]
- Zhao L, Wu F, Zhou T, et al. Risk factors of skip lateral cervical lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Endocrine 2022;75:351-9. [Crossref] [PubMed]
- Wang W, Ding Y, Meng C, et al. Patient's age with papillary thyroid cancer: Is it a key factor for cervical lymph node metastasis? Eur J Surg Oncol 2023;49:1147-53. [Crossref] [PubMed]
- Sisdelli L, Cordioli MICV, Vaisman F, et al. AGK-BRAF is associated with distant metastasis and younger age in pediatric papillary thyroid carcinoma. Pediatr Blood Cancer 2019;66:e27707. [Crossref] [PubMed]
- Iñiguez-Ariza NM, Bible KC, Clarke BL. Bone metastases in thyroid cancer. J Bone Oncol 2020;21:100282. [Crossref] [PubMed]
- Cellini M, Rotondi M, Tanda ML, et al. Skeletal health in patients with differentiated thyroid carcinoma. J Endocrinol Invest 2021;44:431-42. [Crossref] [PubMed]
- Lu J, Liao J, Chen Y, et al. Risk factor analysis and prediction model for papillary thyroid carcinoma with lymph node metastasis. Front Endocrinol (Lausanne) 2023;14:1287593. [Crossref] [PubMed]


