
Pathology and resection margins following mastectomy prior to immediate breast reconstruction
Introduction
The challenge of histopathological examination is to bring complex, three-dimensional biological structures into a two-dimensional understanding. Though constantly striving to describe and quantify all parameters meticulously, histopathology cannot capture the comprehensive picture of multifaceted tumor biology. Instead, it provides the best possible estimation of the tumor’s true biology. Current treatments are nevertheless based on these estimates, which is also true for handling of close/positive margins based on evidence regarding associations on local recurrence (LR) rates and lesion-to-margin distances.
Skin sparing (SSM) and nipple sparing mastectomies (NSM) aim to surgically remove all breast gland tissue via dissection at the subcutaneous fascia level. The superficial/anterior margin is, therefore, considered an anatomic boundary, and not a true resection margin and the flap is expected to hold only skin and subcutaneous fat and minimal amounts of residual breast gland tissue (rBGT). As such, the nipple base in NSM is considered the only true resection margin. There is no consensus on the appropriate intervention in the case of a close/positive superficial margin in mastectomies and handling varies from no intervention, to intervention strategies based on extrapolating level III evidence on breast conserving surgery (BCS) followed by radiotherapy (RT) (1,2). In addition, the superficial/anterior margins in case of mastectomy may not be reported regardless of the surgical procedure (3,4).
In this review, we emphasize the importance of reporting the status of the superficial/anterior margin and the lesion-margin distance to collect evidence regarding the risk of LRs associated with the superficial margin status, and provide guidelines for management of patients with positive superficial margins. Furthermore, we encourage a multidisciplinary effort for better orientation/marking of specimens to correlate in vivo/ex vivo findings thereby securing re-excision of the right area of positive/close margins after mastectomy.
Anatomy
The breast gland is suspended in a three-dimensional fascia system; the anatomy of which has been described through autopsy studies all the way back to Vesalius (5,6). The superficial fascia parts into a dorsal and a ventral sheet that fuses in the periphery of the breast as the circum-mammary ligament (6). The dorsal sheet constitutes a continuous and well-defined layer separating the breast glandular tissue from the pectoral muscle. The ventral sheet of the superficial fascia (= subcutaneous fascia) is, on the contrary, a delicate and discontinuous structure. Based on a study of breast resection specimens, the ventral sheet is an inconsistent anatomical structure and may be absent in almost half of the patients (7). Both sheets of the fascia are separated from the breast glandular tissue by a layer of fatty tissue of varying thickness. The subcutaneous fascia is further separated from the skin by varying layers of subcutaneous fat (median thickness of subcutis 10 mm; range, 0–29 mm) (8,9). In a study by Beer et al. (7), the minimal distance from breast glandular tissue (beneath the fascia) to the dermis was 0.4 mm in patients where the subcutaneous fascia could be found; a distance from dermis to breast tissue of >5 mm was only encountered in 17% of specimens. This distance shows inter- and intra-personal variation. The extension of the breast tissue may be highly imprecise and breast glands may be found in close proximity to skin adnexal structures in some areas and with larger distance in others. In some women a clear distinction can on the other hand be seen between the compact fibroglandular tissue and the overlying fatty tissue (beneath the subcutaneous fascia). The posterior sheet of the fascia is connected to the ventral sheet of the fascia through vertical suspensory ligaments (Cooper’s ligaments) traversing the glandular tissue posteriorly-anteriorly and anchoring the breast gland to the dermis (10). Accordingly, Cooper’s ligaments may be found below and above the subcutaneous fascia level. Isolated glands or lobules may be present in Cooper’s ligaments and have been documented in the subcutaneous fascia in almost half of patients (7). Lobules may also be present in the papilla in 9% to 17% of nipples (9,11). Removal of the whole breast during mastectomy will therefore inevitably leave small amounts of breast glands in patients, and a small risk of subsequent de novo premalignant or malignant lesions persists.
Pathological evaluation of margins
Histopathological examination of margins in BCS as well as in mastectomies begins with clinical information. The quality of the examination depends on availability of information regarding e.g., size of the lesion, if the lesion is well-defined or not, potential satellite foci and distribution of calcifications as determined by imaging. Unambiguous orientation of the specimen by the surgeon is also crucial for the pathologist. Skin-sparing mastectomies and especially NSM have no unique characteristics or features. It is therefore imperative to highlight e.g., two sutures in the cranial and lateral fields to ensure reliable assessment of margins. Figure 1A-1C shows macroscopical pictures of a nipple-sparing mastectomy as it appears when received fresh and unfixated. The base of the nipple in NSM is often not clearly identifiable after a short period of ischemia and drying of the fresh specimen (Figure 1A), so this also needs marking by the surgeon. The nipple base may also not be clearly visible upon sectioning and formalin fixation further compromises the evaluation. Most optimally, the marking of the base of the nipple is done with 4 sutures. Specific information on the surgical procedure is essential for accurate pathological examination, e.g., if the dorsal sheet of the fascia is present on the specimen. If additional tissue has been resected (after initial mastectomy) in the same procedure, the pathologist needs to know the location of this tissue, if it is not stitched to the mastectomy. Any prior sectioning of the specimen, cautery artefacts and fragmentation before the pathological assessment limits the ability to evaluate the margins.
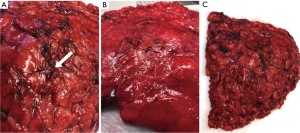
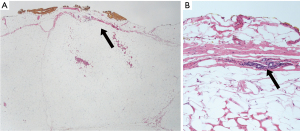
The macroscopical examination is a combination of inspection and palpation. Optimal evaluation of margins begins by inspection of the fresh specimen, though varying national and local guidelines and logistics do not allow for evaluation of fresh specimens in all institutions. Inspecting the fresh specimen, nevertheless, leaves the pathologist with the best impression of the “in vivo” appearance of the breast and the natural boundaries/fascia’s are easier to appreciate. Subsequent fixation creates artefacts due to shrinkage, retraction and deformation (Figure 2). Following mastectomy, the dorsal sheet can easily be visualized macroscopically on the posterior side of the fresh specimen (Figure 1B) and may also be visible microscopically. However, the subcutaneous fascia (ventral sheet) is rarely visible macroscopically; mainly due to varying amounts of subcutaneous fatty tissue covering the surface of the specimen (Figure 1C). Microscopically, the subcutaneous fascia is difficult to appreciate due to the fascia being very thin (often less than 10 µm) and discontinuous (Figure 3A,3B). Often, the fascia cannot be verified microscopically. The impression of the fascia on the ex vivo specimen may contrast with the surgical impression of an avascular plan separating the subcutaneous fat and the breast tissue. During macroscopical evaluation and grossing, the superficial margin of the specimen may be inked, sometimes in several different colors to maintain the orientation (Figure 4A-4D), but margins can also be identified by submitting them into specific cassettes (Figure 5A-5C). Hereafter, the mastectomy is sectioned following national and/or local guidelines, based on fresh or fixated specimens. In Denmark, the Danish Breast Cancer Group (DBCG) have guidelines for imaging, pathology, surgery and RT for patients with breast cancer and ductal carcinoma in situ (DCIS) and non-classical lobular carcinoma in situ (LCIS). There is a high adherence to the national guidelines across Denmark. Institutional and personal preferences may, however, lead to varying grossing procedures similar to variations due to surgeon preference and surgical technique. In general, the whole mastectomy will be sectioned in thin slices for examination. Smaller sections from the lesion will be secured for microscopic analysis as well as any additional areas that are macroscopically and/or palpably distinct from normal breast tissue. These sections also include areas representing the macroscopically closest lesion-to-margin distance.


Sampling from margins can be performed using either tangential sections or perpendicular sections (Figures 4A,4D,5A,5B). The tangential approach is comparable to peeling an orange, which allows for examination of a large area of the surface of the margin. Due to the uneven surface of the mastectomy, the sections chosen for microscopy may, however, vary in thickness (from 2–3 mm). As such, using the tangential approach can lead to misclassification of the exact distance from lesion to the inked margin. Estimation of distance to margin (e.g., “on ink”, <1 mm, >2 mm) can, however, be obtained through deep level sectioning. On the other hand, the perpendicular approach allows measurement of the exact lesion-to-margin distance. However, this approach relies on visualization of smaller, representative areas of the margin of interest and <1% of the margin is expected to be examined (12). The choice of approach varies between pathologists and between specimens. and may be combined if preferred, and it is in general not regulated by guidelines. A survey among Danish breast pathologists, showed that it is primarily perpendicular sections that are taken in areas of interest. Studies of BCS have shown a higher proportion of reported positive margins with the tangential approach, which may partly be due to its limited ability to discriminate close and involved margins (13,14). Similarly, the guidelines may not state how many sections should be sampled, and if sections should be taken from (potentially unaffected) tissue, which is not in the vicinity of the lesion (e.g., from all 4 quadrants) (15). The use of whole mount/large section slides may assist in creating a better view of the lesion, the 3D-architecture and relation to margins (12).
The microscopic analysis relies on the representative sections sampled during grossing. Danish guidelines recommend verification of the margins if they are macroscopically estimated to be less than 15 mm (16). Microscopically, the appearance of breast glandular tissue can easily be distinguished from sporadic glands present in Coopers ligaments. However, the pathologist should be careful not to regard grooves in the uneven fatty tissue as the true surface, when measuring distances from lesions to the superficial margin. Focal resection in the actual breast glandular tissue is not an infrequent finding in SSM/NSM specimens; a finding easily distinguishable from the presence of focal diminutive glands in e.g., Coopers ligaments or subcutaneous fascia (Figure 3B). The distance to closest margin is routinely reported, but in some institutions, the distance to the deep and/or superficial margins may not be reported. Most national guidelines do not provide specific guidance on handling of the superior/anterior margin of mastectomies (4,15-19). In the College of American Pathologists (CAP) guidelines and guidelines of The Royal College of Pathologists (4,19), the synoptic report is recommended to include distance to margin including specification of which margin, also including the deep/posterior and superficial/anterior margins. In the Danish DBCG surgical guidelines, the surgical procedure is, however, considered radical even if there is “tumor on ink” at the superficial margin as long as the surgeon has reported dissection along the superficial fascia (16).
The CAP guidelines recommend including the extent of involvement defined as unifocal (1 focal area of carcinoma at the margin), multifocal (2 or more foci of carcinoma at the margin) or extensive [carcinoma present at the margin over a broad front (>5 mm)] (4), but there is no evidence for these arbitrary categories.
Risk of leaving residual breast tissue
In general, the studies attempting to measure rBGT are limited in number and characterized by using highly varying methodology.
Recent postoperative magnetic resonance imaging (MRI) based studies have shown a higher risk of leaving rBGT in SSM/NSM as compared with simple mastectomy (20,21) with higher likelihood in risk-reducing mastectomies compared with therapeutic procedures. In the study by Woitek et al., rBGT was found in 20% of patients (2.8% after total mastectomy, 13.2% after SSM and 51% after NSM). The finding of rBGT was significantly higher in NSM than in SSM (P=0.003), but with no difference in regard to the location of rBGT (P=0.305). The risk of leaving residual breast glandular tissue has, in general, been described as greatest in the subareolar area and in the upper outer quadrant (20,22). Based on histopathological findings, several earlier studies have described an association between flap thickness and the presence of rBGT (23-25). In the MRI studies, a mean flap thickness of 13.2±9.2 mm (range, 2–39 mm) (20) and 12.1 mm (range, 0–73 mm) (21) was found. The flap thickness was thinnest in the central areas (9.6 mm) and thickest in the periphery (23.2 mm) (20). In support of the histological studies, an association with flap thickness and rBGT was reported on MRI by Giannotti (21), whereas such an association could not be found by Woitek (20).
In the prospective SKINNI trial, systematic biopsies after SSM and NSM were taken from 14 locations in the flap, followed by histological verification of the presence of rBGT in 1,844 biopsies from 160 patients. rBGT was found in 51.3% of skin-flaps and was found to be significantly associated with type of surgery (68.9% NSM vs. 40.4% SSM, P<0.001). The amount of rBGT depended on patient anatomy and a varying distance from breast tissue to margin was found within the same breast (range, 0–10 mm), when measured by the pathologists (26). The presence of rBGT was also dependent on the individual surgeon (P<0.001); all of whom were skilled surgeons with high surgical volumes (>50 surgeries/year). These findings contrast to those observed by Woitek et al., who did not find an association with the individual surgeon (20). Neither Woitek, nor Papassotiropoulos could confirm the assumption that body mass index (BMI) was associated with increased rBGT (20,26). Limited view during surgery in relation to incision type has also been assumed to influence the risk of leaving rBGT. Nonetheless, while a periareolar incision was almost exclusively used in SSM, an association with rBGT and incision type was not found in the SKINNI trial (26).
Based on the current data, mastectomy type and indication influences the risk of rGBT. Factors such as varying thickness of subcutaneous fat layer within the same breast, surgical experience and younger patient age may contribute to a resection that may—at least focally—be below the fascia level and in the actual glandular tissue (Figure 6). Since a larger surface is left in SSM/NSM than in simple mastectomies, there is likely to be more residual breast tissue. The importance of rBGT in terms of risk of developing a subsequent malignancy and the impact of age-dependent involution on this risk are unknown.

Risk of LR after mastectomy and spatial location of recurrences
The risk of loco-regional recurrence (LRR) after mastectomy is low, 2–5% at 10 years, though earlier studies have shown higher LRR risks (up to 20%), e.g., in patients with DCIS. In a recent systematic review of 21 studies (including 6,901 patients) treated with mastectomy (cancer or DCIS), risk of LR was found to be 3.5% with a median time to LR of 26 months (range, 1–169 months) (27).
In a systematic review of 34 studies including 34,833 patients treated with mastectomy for cancer or DCIS, a positive (tumor on ink) or close (<2 mm) margin was in multivariate analysis (MVA) associated with a greater risk of LR [hazard ratio (HR) =2.29 (1.35–3.89) (on ink) and HR =2.96 (2.20–3.98) (<2 mm), respectively] (1). The risk was even more pronounced in SSM in subgroup analysis with an adjusted HR of 3.40 (1.90–6.20) for LR, if there was positive margin with tumor on ink. The review further proved an association between distant disease-free survival and positive margins after mastectomy in MVA [HR =1.53 (1.03–2.25) (on ink)]. In the included studies reporting on multivariate models’, the factors considered included molecular subtype and use of adjuvant therapy (RT and chemotherapy), but information on e.g., BMI or preoperative breast volume was not reported. In addition, a study by Bernstein-Molho and colleagues, investigated rates of LR in BReast CAncer Gene (BRCA) mutation carriers with breast cancer according to the type of surgical procedure (28). They found that those with early stage tumors (T1–2, N0) who underwent immediate breast reconstruction (IBR), without postmastectomy RT (no indication for RT) had higher rates of LR compared with BRCA carriers, who had more advanced disease stage and underwent breast conserving therapy (BCS with RT) or IBR and postmastectomy RT. The cumulative LR rate was 11.8% in the IBR non-RT cohort compared with 0 of IBR-RT group (P=0.01) and 4.7% in the BCS-RT group (P=0.06). As many of the LR occurred within the first 2 years of follow-up, the authors suggested that residual tumour foci and not only rBGT might be responsible for high LR rates in this population.
The spatial location of LR after mastectomy has been studied in a systemic review of the literature by Kaidar-Person et al. (27). The authors concluded that 82% of LR were located in the skin/subcutis and only 18% at the pre-pectoral area. In studies reporting relation between the tumor bed location and LR, 80% of LR were found to be located near the primary tumor bed. After SSM/NSM, LR were exclusively found to be located in the skin/subcutis. These findings suggests that residual tissue/disease has been left at time of surgery. Since superficial margin status in SSM/NSM may not be acted upon and hence not reported, the risk of LR may be underestimated. These results, nevertheless, emphasize the importance of the anterior/superficial margin status and the need to report these margins.
Considerations on how to report and handle a positive margin
After surgery, if a positive margin is reported, the specific area may be difficult to locate during histopathological examination of the specimen, but even more difficult to relocate in the patient; now having a reconstructed breast. Use of photo-documentation during grossing may help pinpoint the area of interest in the case of close margins, but may not help identify the corresponding area in a patient subsequently. Similarly, the insertion of a clip in the patient to indicate areas of concern may not help the pathologist, and so resecting more tissue from these areas during primary surgery is recommended instead. Performing frozen sections from areas of concern during surgery may be used to guide the decision of resecting further tissue in the area. This approach is used in some institutions including Federal University of Goias and the Araújo Jorge Hospital, from Goiás Anticancer Association, in Brazil (personal communication with breast surgeon Ruffo Freitas-Junior). The advantage of this perioperative evaluation is, however, also time consuming, and from a technical perspective, it should be emphasized that the morphology on frozen sections may be suboptimal, and may compromise the distinction between DCIS and e.g., simple ductal hyperplasia.
If the surgeon is uncertain of the location of the positive margin, this may impede further surgery and treatments. Importantly, radiation planning may be hampered as well, as it will be impossible to assure good coverage to the high-risk region. This may result in the need for an additional boost dose to the location of the positive margin. In addition, postmastectomy radiation after IBR leads to significant complications, especially in case of an implant base reconstructions (29). A bolus (tissue equivalent) may be needed to assure coverage of the superficial volumes of the reconstructed breast (30-32), and an additional radiation boost to increase local control (33). These additional treatments are associated with increased acute and late radiation-related toxicities, leading to implant/reconstruction loss without significant improvement of LRs or disease control. A radiation boost has been associated with postoperative infections, skin necrosis, and implant exposure. In case of implant reconstruction patients, the boost was independently associated with increased risk of implant failure (33). Therefore, preoperative planning of appropriate surgery is mandatory to avoid that positive/close margins are the only indication for radiation in these patients.
Handling of a close/involved margin in SSM/NSM demands a well-functioning multidisciplinary communication, but it is equally important for the surgeons and pathologists to constantly communicate on the quality of the individual procedures—even in patients with negative margins. This involves quality control and an honest communication on whether the resection is in fact in the level between the subcutaneous fat and the breast glandular tissue. It is of great importance that the pathologists respect that the subcutaneous fascia may be easily appreciated by the surgeons during surgery, though not easily seen during grossing. It is equally important, that the surgeons acknowledge that microscopically verified breast tissue on the ink, not related to isolated glandular structures in Coopers ligaments or sporadically present in the fatty tissue, unequivocally indicate a resection below the fascia level. We, therefore, suggest that surgeons and pathologists work in tandem to consult on the presence of glands in the different quadrants, periareolar area etc., thereby optimizing the quality of their surgical technique.
In the study by Al-Himdani et al. (3) of 577 breast cancer patients undergoing mastectomy at a single institution, the authors concluded that failure to re-excise in cases of margins with a distance below 1 mm led to an unacceptably high LR rate [adj. HR =2.83; 95% confidence interval (CI): 1.7–4.73)] after SSM. The authors further stated that they, based on these results, prospectively changed their institutional practice to obtain negative margins by re-excision “despite the embarrassment to the surgeon at explaining the issues to the patient”.
Conclusions
Current evidence shows that risk of LR after mastectomy is associated with margin status and that the vast majority of LR are located at the anterior/superficial margin especially in SSM and NSM. Anatomical and histopathological studies have shown the subcutaneous fascia to be very thin, discontinuous and absent in almost half of patients. As such, the subcutaneous fascia may not always be a reliable plane for distinguishing subcutaneous fat and breast tissue. Unwaveringly considering the superficial margin on mastectomies as anatomical boundaries is, therefore, a conception that erroneously may lead to underestimation of the risk of LR after mastectomy.
Based on the current data, we strongly advocate for the continuous reporting of the status of the superficial/anterior margin with exact measurement of lesion to margin distance, and preferably including information on extent of involvement. We further recommend that the pathologist include a note whenever presence of normal fibroglandular tissue is observed at the inked margin for continuous quality assurance for the surgeon.
Acknowledgments
We are very grateful to the breast pathologists that assisted us in understanding national guidelines and to those who provided us with information on their daily routine in handling of mastectomies: Gabor Cserni, Henrik Kiær, Helle Knudsen, Aniko Kovacs, Anne-Vibeke Lænkholm (and the dept. in Roskilde), Elena Provenzano, Nelson Fuentes Martinez, Anne Roslind-Nissen, Elisabeth Specht Stovgaard, Maj-Lis Talman, Pernille Vahl, Jeremias Wohlschläger. A special thanks to breast surgeon Ruffo Freitas-Junior (Head of Program of Mastology of Federal University of Goias and former president of the Brazilian Society of Mastology) for discussions on how to improve the ex and in vivo correlation of a close margin.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard, C. Andrew Salzberg and Jørn Bo Thomsen) for the series “Hot Topics in Breast Reconstruction World Wide” published in Gland Surgery. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-407/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-407/coif). The series “Hot Topics in Breast Reconstruction World Wide” was commissioned by the editorial office without any funding or sponsorship. T.T. reports receiving research grant from Novo Nordic Foundation (2.1 mill DKr), payment or honoraria for lecture from Onkologisk tidskrift, payment or honoraria from Pfizer for educational material for oncologists in training, and holding unpaid positions in the Pathology Commitee in Danish Breast Cancer Group. None of the above relationships are related to the current manuscript. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The macroscopical and microscopical pictures from the mastectomies are fully anonymized and not personally attributable. Photographing of the specimens have not affected the diagnostic procedure and does not warrant informed consent from the patients from whom they originate.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bundred J, Michael S, Bowers S, et al. Do surgical margins matter after mastectomy? A systematic review. Eur J Surg Oncol 2020;46:2185-94. [Crossref] [PubMed]
- Ang SC, Tapia G, Davidson EJ, et al. Positive anterior margins in breast conserving surgery: Does it matter? A systematic review of the literature. Breast 2016;27:105-8. [Crossref] [PubMed]
- Al-Himdani S, Timbrell S, Tan KT, et al. Prediction of margin involvement and local recurrence after skin-sparing and simple mastectomy. Eur J Surg Oncol 2016;42:935-41. [Crossref] [PubMed]
- College of American Pathologists. CAP Cancer Protocol Invasive Breast Cancer. Available online: https://documents.cap.org/protocols/cp-breast-invasive-18protocol-4100.pdf
- Vesalius A. De Humani Corporis Fabrica. Palo Alto, CA, USA: Octavo Corp.; 1543.
- Rehnke RD, Groening RM, Van Buskirk ER, et al. Anatomy of the Superficial Fascia System of the Breast: A Comprehensive Theory of Breast Fascial Anatomy. Plast Reconstr Surg 2018;142:1135-44. [Crossref] [PubMed]
- Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002;94:1619-25. [Crossref] [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [Crossref] [PubMed]
- O’Connell RL, Rusby JE. Anatomy relevant to conservative mastectomy. Gland Surg 2015;4:476-83. [PubMed]
- Cooper A. On the anatomy of the breast. London: Longman, Orme, Green, Brown, and Longmans; 1840.
- Stolier AJ, Wang J. Terminal Duct Lobular Units are Scarce in the Nipple: Implications for Prophylactic Nipple-Sparing Mastectomy. Ann Surg Oncol 2008;15:438-42. [Crossref] [PubMed]
- Tabár L, Dean PB, Lee Tucker F, et al. Can we improve breast cancer management using an image-guided histopathology workup supported by larger histopathology sections? Eur J Radiol 2023;161:110750. [Crossref] [PubMed]
- Wright MJ, Park J, Fey JV, et al. Perpendicular inked versus tangential shaved margins in breast-conserving surgery: does the method matter? J Am Coll Surg 2007;204:541-9. [Crossref] [PubMed]
- Moo TA, Choi L, Culpepper C, et al. Impact of margin assessment method on positive margin rate and total volume excised. Ann Surg Oncol 2014;21:86-92. [Crossref] [PubMed]
- Cserni G, Francz M, Járay B, et al. Pathological Diagnosis, Work-Up and Reporting of Breast Cancer 1st Central-Eastern European Professional Consensus Statement on Breast Cancer. Pathol Oncol Res 2022;28:1610373. [Crossref] [PubMed]
- Patologiprocedurer og molekylærpatologiske analyser ved brystkræft. DBCG. Version 1.1. [cited 2021 Feb 21]. Available online: https://www.dmcg.dk/kliniske-retningslinjer
- Kvalitetsbilaga för bröstpatologi (KVAST-bilaga). Available online: https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/vardprogram/kvalitetsdokument-for--patologi/
- Leitlinienprogramm Onkologie. Interdisziplinäre S3-Leitlinie für die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Mammakarzinom_4_0/Version_4.4/LL_Mammakarzinom_Langversion_4.4.pdf
- The Royal College of Pathologists. Breast histopathology synoptic report. Available online: https://www.rcpath.org/search-results.html?q=Breast histopathology synoptic report
- Woitek R, Pfeiler G, Farr A, et al. MRI-based quantification of residual fibroglandular tissue of the breast after conservative mastectomies. Eur J Radiol 2018;104:1-7. [Crossref] [PubMed]
- Giannotti DG, Hanna SA, Cerri GG, et al. Analysis of Skin Flap Thickness and Residual Breast Tissue After Mastectomy. Int J Radiat Oncol Biol Phys 2018;102:82-91. [Crossref] [PubMed]
- Kaidar-Person O, Boersma LJ, Poortmans P, et al. Residual Glandular Breast Tissue After Mastectomy: A Systematic Review. Ann Surg Oncol 2020;27:2288-96. [Crossref] [PubMed]
- Torresan RZ, Cabello dos Santos C, Brenelli H, et al. Residual glandular tissue after skin-sparing mastectomies. Breast J 2005;11:374-5. [Crossref] [PubMed]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Ho CM, Mak CK, Lau Y, et al. Skin involvement in invasive breast carcinoma: safety of skin-sparing mastectomy. Ann Surg Oncol 2003;10:102-7. [Crossref] [PubMed]
- Papassotiropoulos B, Güth U, Chiesa F, et al. Prospective Evaluation of Residual Breast Tissue After Skin- or Nipple-Sparing Mastectomy: Results of the SKINI-Trial. Ann Surg Oncol 2019;26:1254-62. [Crossref] [PubMed]
- Kaidar-Person O, Poortmans P, Offersen BV, et al. Spatial location of local recurrences after mastectomy: a systematic review. Breast Cancer Res Treat 2020;183:263-73. [Crossref] [PubMed]
- Bernstein-Molho R, Laitman Y, Galper S, et al. Locoregional Treatments and Ipsilateral Breast Cancer Recurrence Rates in BRCA1/2 Mutation Carriers. Int J Radiat Oncol Biol Phys 2021;109:1332-40. [Crossref] [PubMed]
- de Boniface J, Coudé Adam H, Frisell A, et al. Long-term outcomes of implant-based immediate breast reconstruction with and without radiotherapy: a population-based study. Br J Surg 2022;109:1107-15. [Crossref] [PubMed]
- Kaidar-Person O, Dahn HM, Nichol AM, et al. A Delphi study and International Consensus Recommendations: The use of bolus in the setting of postmastectomy radiation therapy for early breast cancer. Radiother Oncol 2021;164:115-21. [Crossref] [PubMed]
- Dahn HM, Boersma LJ, de Ruysscher D, et al. The use of bolus in postmastectomy radiation therapy for breast cancer: A systematic review. Crit Rev Oncol Hematol 2021;163:103391. [Crossref] [PubMed]
- Nichol A, Narinesingh D, Raman S, et al. The Effect of Bolus on Local Control for Patients Treated With Mastectomy and Radiation Therapy. Int J Radiat Oncol Biol Phys 2021;110:1360-9. [Crossref] [PubMed]
- Naoum GE, Salama L, Ho A, et al. The Impact of Chest Wall Boost on Reconstruction Complications and Local Control in Patients Treated for Breast Cancer. Int J Radiat Oncol Biol Phys 2019;105:155-64. [Crossref] [PubMed]



