Radiofrequency ablation as a novel modality in Ecuador for treating toxic thyroid nodules: a case series
Highlight box
Key findings
• Radiofrequency ablation (RFA) was a feasible and safe alternative for treating autonomously functioning thyroid nodules (AFTNs).
• The nodular volume reduced significantly after RFA treatment over time (P<0.001).
• The thyroid-stimulating hormone normalized after RFA and they did not use hyperthyroid medications.
What is known and what is new?
• The euthyroid restorage at 12 months after a single session of RFA.
• After a single RFA session, the volume reduction at 6 months was 70% (standard deviation: 6.79; P=0.016).
What is the implication, and what should change now?
• RFA may be more helpful in single AFTN than toxic multinodular goiter.
• Small nodules (<12 mL) responded better to RFA than medium-sized nodules (>12 mL).
Introduction
An autonomously functioning thyroid nodule (AFTN) is typically benign and makes up roughly 5% of all thyroid nodules. It presents with a wide range of clinical features, with the potential to progress from normal thyroid function to hyperthyroidism. Antithyroid drugs (ATDs) may be employed to restore a euthyroid state. The primary treatments for AFTN include surgery and radioiodine (RAI), according to the initial approach. However, some patients may opt out of these options or face contraindications. Minimally invasive techniques like radiofrequency ablation (RFA) have shown volume reduction (VR) and euthyroid restoration in treating AFTN (1).
RFA is widely acknowledged as an effective treatment for non-functioning benign thyroid nodules, primarily aimed at alleviating compressive symptoms (2). European guidelines advocate for RFA in young patients with small AFTN, emphasizing a higher likelihood of restoring normal thyroid function and avoiding irradiation. Furthermore, an international consensus suggests that RFA is most suitable for patients with small nodules (≤3 cm) and contraindications to RAI or surgery (3). In Europe, an RFA applicator costs between 500 and 1,000 euros, and the procedure is typically performed in an outpatient setting, taking 15 to 40 minutes (4). While RFA has recently been introduced in the U.S. and Latin American countries, Ecuador began using it in 2019 for patients with AFTN and papillary thyroid carcinoma (PTC).
Our study aims to describe the demographic characteristics and clinical outcomes following the first cohort of patients with AFTN who underwent RFA in Ecuador. We present this article in accordance with the PROCESS reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-425/rc) (5).
Methods
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients to publish this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Participants
This case series occurred at the Institute of Thyroid and Head and Neck Diseases (ITECC), a private referral center for individuals with thyroid nodules in Quito, Ecuador. From July 2022 to May 2023, the study included eight patients who underwent RFA for AFTN under the care of a head and neck surgeon (C.G.).
Pre-ablation assessment
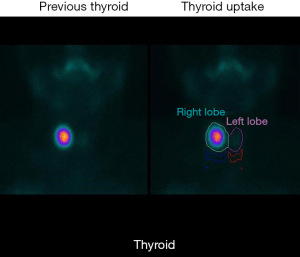
Before treatment, comprehensive assessments were conducted, encompassing blood tests, thyroid function tests, coagulation tests, and imaging evaluations. Ultrasound scans provided detailed information on nodule characteristics such as size, location, margin, shape, echogenicity, calcification, and vascularity. Thyroid scintigraphy was performed for all patients to visually depict functional thyroid tissue based on the selective uptake of radionuclides (Figure 1). Nodule volume was determined using the American Thyroid Association (ATA) formula (6). Nodules were categorized as solid, predominantly solid, predominantly cystic, or cystic based on their cystic-to-solid ratio (7), with mixed nodules defined as having a solid component between 30% and 70%.
Ablation technique
RFA, administered by a head and neck surgeon with 3 years of RFA experience, was carried out using local anesthesia (2% lidocaine without epinephrine) as an outpatient procedure. After cleansing with povidone-iodine, a needle with an 18-gauge, 7-mm active tip size electrode was positioned within the nodule using a long-axis trans-isthmic approach. Nodules were then subjected to ablation using the moving-shot technique.
Follow-up evaluation
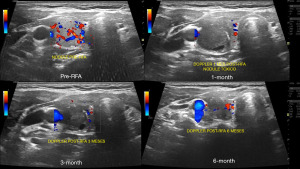
Patients were monitored at 1, 3, and 6 months post-RFA (Figure 2). The evaluation included an assessment of hyperthyroid symptoms and the use of antithyroid medication. Ultrasound neck scans and laboratory tests were mandatory at each follow-up visit. The ATA volume calculator determined the treated nodules’ VR.
Analysis and statistics
Statistical analysis utilized the R program. Distribution normality was assessed visually and through the Shapiro-Wilks test. For continuous variables, the medians and interquartile ranges (IQRs) were calculated. Categorical variables were presented by frequency (percentage). The pre- and post-RFA VR changes, thyroid-stimulating hormone (TSH), and free thyroxine (fT4) laboratory values were assessed using a paired t-test. Cohen’s d value was employed to gauge the magnitude of mean differences as small, medium, or large.
Case presentation
Demographic characteristics
Table 1 shows the clinical characteristics of the patients with AFTNs who underwent RFA. All the patients were females (n=8). The mean age was 41.63 years [standard deviation (SD): 14.97 years]. Five patients who had symptomatic hyperthyroidism (tachycardia, high blood pressure, or weight loss) were treated with hyperthyroid drugs in addition to RFA. Also, three patients had subclinical hyperthyroidism. All the patients had a thyroid scintigraphy pre-RFA (Figure 1).
Table 1
| Variables | Value |
|---|---|
| Sex, n (%) | |
| Female | 8 (100.0) |
| Age at diagnosis (years), mean (SD) | 41.63 (14.97) |
| Residence, n (%) | |
| Coast | 3 (37.5) |
| Highland | 5 (62.5) |
| Employment, n (%) | |
| Domestic chores | 1 (12.5) |
| Student | – |
| Labor | 7 (87.5) |
| Education level, n (%) | |
| High school | 1 (12.5) |
| University | 7 (87.5) |
| BMI (kg/m2) | |
| Mean (SD) | 25.13 (4.34) |
| Normal, n (%) | 3 (37.5) |
| Overweight, n (%) | 4 (50.0) |
| Obesity, n (%) | 1 (12.5) |
| Nodule composition, n (%) | |
| Solid | 3 (37.5) |
| Predominantly solid | 5 (62.5) |
| Laterality, n (%) | |
| Right lobe | 5 (62.5) |
| Left lobe | 3 (37.5) |
| Isthmus | – |
| Methods of detection, n (%) | |
| Hyperthyroidism | 5 (62.5) |
| Subclinical hyperthyroid | 3 (37.5) |
| Hyperthyroid drugs before RFA, n (%) | |
| None | 3 (37.5) |
| Propylthiouracil | 4 (50.0) |
| Propylthiouracil + beta-blocker | 1 (12.5) |
| Methimazole | 0 (0.0) |
| Radioactive iodine | 0 (0.0) |
SD, standard deviation; BMI, body mass index; RFA, radiofrequency ablation.
The median follow-up time was 10 months (IQR, 7−12 months). All the patients required one session of RFA, and most AFTNs were located on the right side (62.5%). Nodules were solid (37.5%) or predominantly solid (62.5%).
VR
Table 2 shows that the overall median baseline volume of the AFTNs before RFA was 5.27 mL (IQR, 0.70−9.66 mL). After ablation, the 1-, 3-, and 6-month median volumes were 2.25 (SD: 1.67; P<0.12), 1.28 (SD: 1.1; P=0.013), and 1.37 (SD: 1; P=0.23) mL, respectively. Moreover, the results showed that the overall nodular volume reduced significantly after RFA treatment over time (P<0.001) (Figure 3).
Table 2
| Variables | Baseline | 1 month | 3 months | 6 months |
|---|---|---|---|---|
| Longest tumor diameter | ||||
| Mean [SD], mm | 30.38 [12.22] | 25 [8.86] | 19.5 [5.54] | 14.3 [4.04] |
| N | 8 | 5 | 6 | 3 |
| Tumor volume | ||||
| Mean [SD], mL | 5.27 [3.64] | 2.25 [1.67] | 1.28 [1.1] | 1.37 [1] |
| N | 8 | 5 | 6 | 2 |
| P | – | <0.12 | 0.013 | 0.23 |
| VR | ||||
| Mean [SD], % | – | 45.8 [28.89] | 75.1 [13.1] | 69.7 [6.8] |
| N | – | 5 | 6 | 2 |
| TSH | 1.22 | 2.86 | 1.76 | 1.88 |
RFA, radiofrequency ablation; SD, standard deviation; VR, volume reduction; TSH, thyroid-stimulating hormone.
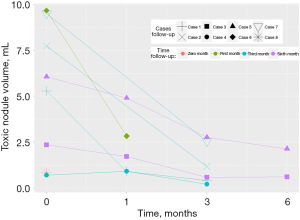
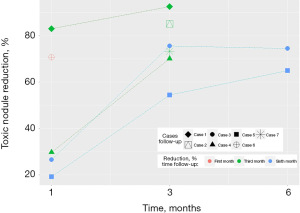
Patients undergoing RFA experienced a large and statistically significant difference in the percentage of toxic nodule reduction (%) over time. Figure 4 shows that in the first month, percentage reduction data was reported for five patients (mean =45.8%; 19.2–83.0%). In the third month of follow-ups, data from six patients were reported (mean =75.1%; 54.4–92.5%; SD: 13.1; P=0.029), and in the sixth month, two patients were reported (mean =69.7%; 64.9–74.5%; SD: 6.79; P=0.016).
The active ablation time, power, and energy delivered by the procedure were 6 minutes 59 seconds (SD: 5 minutes 7 seconds), 38.57 W (SD: 6.48 W; range, 35–60 W), and 9.28 kJ (SD: 7.03 kJ), respectively.
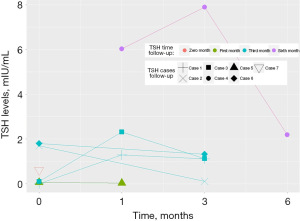
TSH reduction
Figure 5 shows that patients who underwent RFA experienced a small but not statistically significant difference in the increase in TSH levels (mIU/mL) after 1, 3, and 6 months post-RFA (mean =3,312 mIU/mL; SD: 3,001 mIU/mL).
We did not have complications after the RFA. After RFA, patients reported improved hyperthyroidism symptoms and quality of life. Also, they did not need more RFA sessions, which made them happy.
Discussion
This study found that all isolated AFTNs were ablated in a single session, and all patients had significant reductions in the volume of nodules, improved hyperthyroidism symptoms, and reduced use of ATD medication.
The European, AHNS international, NASOIE, and ATA guidelines all state that RFA is an option for AFTN—with the European guidelines being the most conservative. Moreover, the Korean RFA guidelines advocate using RFA for AFTNs following a biopsy (6). This recommendation extends to patients with cosmetic concerns or hyperthyroid symptoms, irrespective of the nodule’s size. In contrast, a German consensus discourages using RFA for AFTNs with volumes exceeding 12 to 15 mL (7). We followed Korean guidelines to include the patient for RFA. The inclusion criteria of our patients agree with a recent statement of the ATA, citing that a unique skill set, and environment are needed for optimal, safe performance and consistent outcomes (8).
RFA is a new alternative to treat patients with AFTN. The European guideline shows that RFA proves effective in treating thyroid nodules, with a mean VR of 69–78% at 12 months in randomized controlled trials, and long-term clinical efficacy is demonstrated in a 5-year retrospective study, showing a median VR of 67% (4). Regarding the AFTN, the VR and reduction of hyperthyroidism symptoms are used to evaluate the efficacy. In a retrospective study of 20 AFTN, Wang et al. reported a median VR of 88.3% (78.3–96.2%) with a euthyroid restoration rate of 75.0% at 12 months after a single session of RFA (9). Cappelli et al. reported a VR of 72.9% and thyroid function normalization of 94.1% in 17 patients with toxic nodules at 12 months after a single session of RFA (10). One recent meta-analysis that included 10 AFTN studies showed a pooled VR of 76.5% and a normalization of thyroid function rate of 61.7% for RFA approaching toxic AFTN, with sample sizes ranging from 9 to 44 and follow-up periods varying from 6 to 24 months (11). Our results align with previous studies with a VR at 6 months of 70% (SD: 6.79; P=0.016) after a single RFA session.
Cesareo et al., in a prospective study of 24 AFTNs, found that small nodules (<12 mL) responded better to RFA than medium-sized nodules (>12 mL) (12). All our AFTNs had a volume of less than 12 mL.
Moreover, RFA may be more helpful in single AFTN than TMG. van der Meeren, in a retrospective study of 36 patients with isolated AFTN and 12 TMGs with 1-year follow-up post-RFA, showed a cure rate of 72% vs. 25% in TMG (P=0.004) (13). This study reported that the severity of hyperthyroidism [higher baseline TSH and a lower free triiodothyronine (fT3)] and kJ/mL delivered during RFA (>2.1 kJ/mL) predicts a cure. Moreover, this study demonstrated that the efficacy of RFA was nearly three times higher in solitary toxic adenoma than in TMG. Our study was focused on solitary AFTN.
This study has several limitations. The sample size needs to be increased to have significant results. Furthermore, we could only provide information about the outcomes for a short follow-up since the RFA started recently in our country. Despite these limitations, the strength of this study is that the RFA has allowed treating patients with AFTN who are contraindicated to other therapies.
Conclusions
In this first report from Ecuador, we found that RFA was a feasible and safe alternative for treating AFTNs. Long-term data are needed to evaluate the prediction of treatment success with the association between energy delivered per mL, the severity of hyperthyroidism, and thyroid volume before RFA.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PROCESS reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-425/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-425/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-425/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pace-Asciak P, Russell JO, Shaear M, et al. Novel Approaches for Treating Autonomously Functioning Thyroid Nodules. Front Endocrinol (Lausanne) 2020;11:565371. [Crossref] [PubMed]
- Kim HJ, Cho SJ, Baek JH, et al. Efficacy and safety of thermal ablation for autonomously functioning thyroid nodules: a systematic review and meta-analysis. Eur Radiol 2021;31:605-15. [Crossref] [PubMed]
- Orloff LA, Noel JE, Stack BC Jr, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck 2022;44:633-60. [Crossref] [PubMed]
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J 2020;9:172-85. [Crossref] [PubMed]
- Agha RA, Sohrabi C, Mathew G, et al. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting Of CasESeries in Surgery (PROCESS) Guidelines. Int J Surg 2020;84:231-5. [Crossref] [PubMed]
- Lee M, Baek JH, Suh CH, et al. Clinical practice guidelines for radiofrequency ablation of benign thyroid nodules: a systematic review. Ultrasonography 2021;40:256-64. [Crossref] [PubMed]
- Feldkamp J, Grünwald F, Luster M, et al. Non-Surgical and Non-Radioiodine Techniques for Ablation of Benign Thyroid Nodules: Consensus Statement and Recommendation. Exp Clin Endocrinol Diabetes 2020;128:687-92. [Crossref] [PubMed]
- Sinclair CF, Baek JH, Hands KE, et al. General Principles for the Safe Performance, Training, and Adoption of Ablation Techniques for Benign Thyroid Nodules: An American Thyroid Association Statement. Thyroid 2023;33:1150-70. [Crossref] [PubMed]
- Wang L, Wang P, Chen Z, et al. Image-guided Thermal Ablation as a Promising Approach to Both Nontoxic and Toxic Autonomously Functioning Thyroid Nodules. Acad Radiol 2023;30:2636-46. [Crossref] [PubMed]
- Cappelli C, Franco F, Pirola I, et al. Radiofrequency ablation of functioning and non-functioning thyroid nodules: a single institution 12-month survey. J Endocrinol Invest 2020;43:477-82. [Crossref] [PubMed]
- Muhammad H, Tehreem A, Russell JO, et al. Radiofrequency Ablation and Autonomous Functioning Thyroid Nodules: Review of the Current Literature. Laryngoscope 2022;132:906-14. [Crossref] [PubMed]
- Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency Ablation on Autonomously Functioning Thyroid Nodules: A Critical Appraisal and Review of the Literature. Front Endocrinol (Lausanne) 2020;11:317. [Crossref] [PubMed]
- van der Meeren MMD, Joosten FBM, Roerink SHPP, et al. Radiofrequency ablation for autonomously functioning nodules as treatment for hyperthyroidism: subgroup analysis of toxic adenoma and multinodular goitre and predictors for treatment success. Eur J Nucl Med Mol Imaging 2023;50:3675-83. [Crossref] [PubMed]