
Pre-pectoral breast reconstruction with tissue expander entirely covered by acellular dermal matrix: feasibility, safety and histological features resulting from the first 64 procedures
Highlight box
Key findings
• Two-stage pre-pectoral breast reconstruction (PPBR) with tissue expander (TE) entirely covered by acellular dermal matrix (ADM) is as effective as ADM-assisted direct-to-implant pre-pectoral reconstruction as concerns complications and tissue regeneration.
What is known and what is new?
• Literature data demonstrate that PPBR performed with implants entirely covered by BRAXON®Fast ADM has low complication rates with the device promoting subcutaneous tissue regeneration, hence the formation of a soft and vascularised peri-capsular tissue.
• Two-stage PPBR performed with TEs entirely covered by BRAXON®Fast shows complication rates fully in line with good clinical practice. In addition, this work proves that such ADM is compatible with the dynamic biological environment that subcutaneous tissue experiences during expansion, and matrix integration, repopulation and vascularization take place. The formed peri-capsular tissue is soft and vascularised.
What is the implication, and what should change now?
• This work enlarges the indications for BRAXON®Fast-assisted PPBR by providing insights on a variation of the technique. More patients can benefit from saving the pectoralis major muscle.
Introduction
Breast reconstruction is a central component of the comprehensive management of breast cancer patients, offering physical and psychological restoration for mastectomised women. Reconstructive procedures have evolved significantly over the years, providing improved outcomes and enhanced patient satisfaction (1). Optimal aesthetic outcomes with long-term functional and clinical stability are reported worldwide with pre-pectoral breast reconstruction (PPBR), turning now from innovation to new gold standard in implant-based reconstructive surgery (2,3).
Acellular dermal matrices (ADMs) have played a pivotal role in enabling pre-pectoral advancement: they allowed subcutaneous implant placement by providing a higher-quality biocompatible interface around the synthetic prosthesis which reduced incidences of capsular contracture by regeneration of subcutaneous tissue (3-8). As a matter of fact, derived from allogeneic or xenogeneic dermal tissue sources, ADMs are processed to remove cellular elements while preserving extracellular matrix (ECM) structural integrity and components (9). As a result, collagen is the major constituent of dermis-derived acellular materials, physiologically designed to provide structural support and facilitate cellular adhesion, migration, and proliferation (10,11). As such, whenever implanted within biological tissues, ADMs act as a three-dimensional scaffold that activates biological healing mechanisms involving cellular infiltration, angiogenesis, and remodelling, leading to the development of a functional neo-tissue. In other words, ADMs promote their own integration into patient’s tissues, creating a coverage that minimizes implant-related foreign body reactions (4,9,12,13).
Over the past 20 years, ADMs’ anti-fibrotic properties have been extensively documented in implant-based breast surgeries, with established improvements of clinical-aesthetic outcomes (14-16). The first successful pre-pectoral reconstruction with the first ADM designed to completely cover an implant, named BRAXON®, described by Berna et al. is one of the present-day example par excellence of the enhancement achieved thanks to these biomaterials (2,3,5,17,18).
In the wake of these results, pre-pectoral procedures are recently beginning to be performed even in two stages. Several levels of ADM coverage have been reported in this setting taking advantage of their properties by applying an ADM directly on the tissue expander (TE). Interestingly, multiple publications report favourable final outcomes, yet none report on a full wrap with xenogeneic ADM on the synthetic expander (19-22). Furthermore, studies investigating ADMs’ integration in such a dynamic frame are scarce, although two-stage techniques offer the significant chance to explore the breast pocket, collecting biopsy specimens at the time of the definitive implant positioning (23,24).
The aim of this study is to share our Unit experience on two-stage pre-pectoral procedure in terms of safety and biological tissues integration using a complete ADM wrap of the TE. We report clinical and histological analyses of reconstructions performed with BRAXON®Fast, a xenogeneic dermal matrix specifically designed to completely isolate the synthetic material in the breast pocket. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-432/rc).
Methods
Patients and data
A retrospective analysis was conducted on 70 patients (a total of 84 breasts) treated for breast cancer and two-stage pre-pectoral reconstruction at our Breast Unit (Azienda Ospedaliero Universitaria Careggi, University of Florence, Italy) from March 2021 to May 2023. At our Institution, the two-step procedure is offered to patients who would benefit from the sparing of the pectoralis major muscle but who are not good candidates for direct-to-implant reconstructions because of obesity, previous radiotherapy treatment, hypertension, neo-adjuvant chemotherapy, and undergoing a skin-sparing procedure (because of nipple-areola complex removal). In our work, we included these patients, plus those who had small to moderate-sized breasts (<500 g) and were wishing for a larger cup. Patients with more than two comorbidities and with TNM status >4 were not deemed suitable for such procedure. Therefore, patients who satisfied the listed criteria received a mesh/matrix-covered pre-pectoral TE breast reconstruction. no more, small to moderate-sized breasts. For the purpose of this work, in order to obtain homogeneity of the analysed population, patients who underwent two-stage pre-pectoral reconstruction with TE wrapped in ADM or meshes other than BRAXON®Fast (Decomed® S.r.l., Venice, Italy) were excluded from the analysis. Demographic data such as BMI, smoking habit, neo-adjuvant and adjuvant therapies, comorbidities, previous breast surgeries, hospital stay, and surgical details were recorded on the institutional database. Reconstructive outcomes and complications such as seroma, dehiscence, infection, hematoma, TE rupture and failure were recorded and classified as early or late depending on the timing of occurrence (before or after three months from surgery respectively).
In five cases, at second stage operation, when patients returned into the operating room for TE removal and definitive implant placement, a 1.5 cm × 1.5 cm square of peri-capsular tissue was sampled for histological investigations, consisting in haematoxylin and eosin (H&E) staining on tissue sections.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Azienda Ospedaliero Universitaria Careggi (No. #16.069_AOUC) and individual consent for this retrospective analysis was waived.
Surgical technique
The surgical technique for two-stage ADM-assisted PPBR is similar to that of direct-to-implant (DTI) ADM-assisted PPBR. Briefly, soon after mastectomy the patient is ready for BRAXON®Fast-wrapped TE implantation. BRAXON®Fast is a pure collagen matrix devoid of preservative or cross-linking agents. It is 0.6 mm thick, and the unique three-dimensional patented design presents a dome-shaped anterior part that can easily adapt to all implants’ silhouettes. The ADM preparation consists in a 5-minute hydration in room temperature sterile solution so that the matrix becomes pliable and can be easily adapted to the TE silhouette. The TE is expanded up to approximately 30-50% of the desired volume with sterile saline solution and is placed inside the ADM. The dome-shaped superior flap and the inferior flat flap of the matrix are sutured together with absorbable 3.0 Vicryl Rapide® interrupted stitches (that will dissolve in little more than 1 month) so that a snug envelope is all around the TE, quite tight in order to prevent TE malrotation but still not completely adherent to the TE itself, to allow for the first month initial expansions. The BRAXON®Fast-TE complex is then positioned in the breast pocket and fixed to the pectoralis major muscle for stability, with 2 or 3 interrupted sutures, once again absorbable even though a little more long lasting and with a 2.0 calibre. Adherence of the ADM to the mastectomy flap, to prevent the formation of dead spaces, can be adjusted by tuning the TE inflation. Nonetheless, skin flaps should always be kept quite loose in order to get an adequate blood flow and less tension on the incision edges, thus taking advantage of a two-stage reconstruction as compared to a DTI. Only one drain is placed around the TE/ADM complex. Patients are discharged with drain and wearing compressive bandages/bra. Drain is removed when the liquid volume in the output bag reaches 30 cc for 2 consecutive days. Patients are suggested to wear the compressive bra for at least one month. A variable number of TE expansions is performed until the desired final breast volume is reached. After a month the stitches of the ADM envelope are dissolved and expansion can be completed as wished, stretching entirely the matrix surface, and creating a complete adherence of TE and ADM on the inner aspect and of ADM and skin flap on the other side. At the time of TE-definite implant substitution lipofilling could be performed.
Statistical analysis
Data are presented as a descriptive analysis of demographical and surgical data, and complications. Data are reported as number, range, mean with standard deviation, median, and percentage.
Results
From March 2021 to May 2023, a total of 55 patients (64 breasts) underwent mastectomy and two-stage ADM-assisted PPBR using BRAXON®Fast at our Institution. Data were retrieved from Institutional database for all patients. Patients were followed-up for an average of 10 months (median follow-up 8.7 months). A summary of demographic and surgical data is reported in Table 1.
Table 1
| Demographics | Values |
|---|---|
| Patients, n | 55 |
| Breasts, n | 64 |
| Follow-up (months), mean ± SD [range] | 10±5.4 [1.5–28.3] |
| Age (years), mean ± SD [range] | 50.7±10 [30–74] |
| BMI (kg/m2), mean ± SD | 23.1±3.8 |
| Hospital stay (days), mean ± SD | 2.4±0.9 |
| Smoking status (per patient), n (%) | |
| Non smokers | 39 (70.9) |
| Current smokers | 10 (18.2) |
| Former smokers | 6 (10.9) |
| Comorbidities (per patient), n (%) | |
| Diabetes | 1 (1.8) |
| Autoimmune diseases | 2 (3.6) |
| Cardio-vasculopathies | 9 (16.4) |
| Hypothyroidism | 5 (9.1) |
| Other comorbidities | 7 (12.7) |
| BRCA1/2mut carriers | 7 (12.7) |
| Surgery type (per breast), n (%) | |
| Therapeutic | 51 (79.7) |
| Prophilactic | 13 (20.3) |
| Type of tumor (per breast), n (%) | |
| DCIS | 10 (15.6) |
| LCIS | 7 (10.9) |
| IDC | 9 (14.1) |
| ILC | 2 (3.1) |
| Mixed | 1 (1.6) |
| Other | 22 (34.4) |
| Mastectomy (per breast), n (%) | |
| Skin/nipple-sparing | 32 (50.0) |
| Skin-sparing | 30 (46.9) |
| Skin-reducing | 2 (3.1) |
| Incisions (per breast), n (%) | |
| Italic-S | 18 (28.1) |
| Elliptical | 29 (45.3) |
| Wise pattern | 4 (6.3) |
| Inframammary fold | 13 (20.3) |
| Therapies, n (%) | |
| Chemotherapy (per patient) | |
| Neoadjuvant | 7 (12.7) |
| Adjuvant | 13 (23.6) |
| Radiotherapy (per breast) | |
| Pre-operative | 3 (4.7) |
| Post-operative | 10 (15.6) |
| Other details (per breast) | |
| Implant volume (cc), mean ± SD | 380±123 |
| Axillary lymphadenectomy, n (%) | 15 (23.4) |
| Previous breast surgery, n (%) | 11 (17.2) |
| Drainage (days), mean ± SD | 18.5±8.0 |
| Lipofilling, n (%) | 7 (10.9) |
SD, standard deviation; BMI, body mass index; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Early complications occurred in 9.4% of the breasts (6 breasts). The most observed were dehiscence and hematoma, each occurred in 2 breasts (3.1%), followed by 1 seroma (1.6%) and 1 infection (1.6%), all conservatively treated without further complications. No skin or nipple-areola complex necrosis were observed. The only 2 (3.1%) late complications recorded were 1 wound infection with dehiscence (1.6%) and 1 expander rupture (1.6%). Both required reintervention and the TE had to be removed, thus reconstructive failure occurred in 2 breasts (3.1%). There was no evidence of capsular contracture especially for those patients with a follow-up longer than 1 and 2 years (15 patients and 3 patients, respectively). In addition, 20% of the breasts underwent radiotherapy, a known risk factor for early onset of capsular contracture. Such complication was not observed in irradiated patients. All complications are reported in Table 2.
Table 2
| Complications | N (%) |
|---|---|
| Early complications | |
| Seroma | 1 (1.6) |
| Dehiscence | 2 (3.1) |
| Infection | 1 (1.6) |
| Hematoma | 2 (3.1) |
| NAC/skin necrosis | 0 |
| Total | 6 (9.4) |
| Late complications | |
| Infected dehiscence | 1 (1.6) |
| TE rupture | 1 (1.6) |
| Capsular contracture | 0 |
| Total | 2 (3.1) |
| Failure | 2 (3.1) |
NAC, nipple-areola complex; TE, tissue-expander.
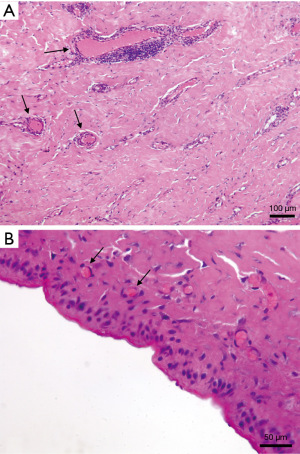
At the time of TE-definitive implant exchange, the peri-implant capsule appeared always soft, elastic, and vascularised (Figure 1), indicating Braxon® successful integration into the surrounding tissues. All tissue samples analysed with H&E revealed presence of blood vessels in the newly formed tissue (Figure 2A,2B). Where the ADM was in contact with the expander a thin layer of synovial metaplasia had formed (Figure 2B).

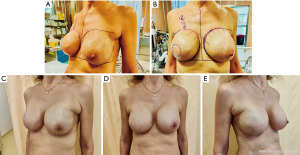
Aesthetic results obtained with this reconstructive technique are reported in Figure 3, both after breast expansion (Figure 3A, right before implant exchange) and after definitive implant placement (Figure 3B).
Discussion
Recent emphasis on personalized breast reconstruction plans considers each patient’s uniqueness, enhancing satisfaction and success rates with a diverse range of techniques and devices (21,25).
One prominent trend in implant-based reconstruction is ADM-assisted pre-pectoral implant placement, performed either in one-stage or two-stage modalities with established major benefits and patient satisfaction. Here we have shown the outcomes on 64 two-stage pre-pectoral reconstructions performed with BRAXON®Fast-covered TEs. The ADM used is of pig origin, it is the only one that allows complete implant coverage and that presents a three-dimensional dome shape on the anterior part which easily allocates various types and dimensions of TEs/implants without the need for time-consuming cutting and sewing required to adapt flat ADMs to curved surfaces (26). In addition, such device demonstrated adipogenic stimulation capacity, thus it is able to boost a more physiological tissue regeneration and replenishing the cells naturally present in the subcutaneous tissue (27).
Decellularized dermis materials enable subcutaneous implants, reducing inflammation and profibrotic signalling in breast capsule development (4,17,24). Based on this rationale, many clinicians have verified improved clinical-aesthetic outcomes with complete ADM wrapping of the silicone prosthesis. Masià et al., for example, reported only a 2.1% capsular contracture rate on 1,450 pre-pectoral procedures, with very natural-looking aesthetic outcomes (2).
Such positive results seem to recur even in radiotherapy settings (28-30) or in obese patients (31) whenever applying a regenerative shell in pre-pectoral surgeries. TEs, like silicone implants, induce a foreign body reaction. Without a suitable bio-active coating, subcutaneous placement can lead to adverse effects (32,33). Accordingly, Chopra et al. reported quite frequent adverse events with plain pre-pectoral TEs, including device dystopia, with recorded rates between 32.4% and 45.9% (34). In another retrospective review of 250 nude pre-pectoral expanders, Salibian et al. documented grade III/IV capsular contracture in 7.6% of cases (35). Likewise, Hammond et al. gived evidence of 21.1% capsular contracture grade III/IV in nineteen revision surgeries following pre-pectoral conversion without ADM within a mean follow-up of 13.8 months (36).
Conversely, data on ADM-wrapped expanders generally reveal lower complication rates. Woo et al. describeb a 10% of adverse events when a nearly complete ADM coverage of the expander is applied, as well as Sigalove reports a total complications rate of 5.9% with expanders fully covered with one or two sheets of acellular dermis (25,29). Interestingly, when only ADM tenting is applied, post-operative clinical profile seems to shift toward slightly increased complication rates (20,37).
The extent of ADM implant coverage is still debated. In our practice, an ADM for complete prosthesis wrap was opted, as mechanical and anti-fibrotic abilities of ADMs have been extensively demonstrated and involving the entire synthetic surface could maximize their action (38,39). Several clinicians have already adopted this strategy, making use of human dermal matrices (21,22,25,38). However, allogeneic dermis is cost-prohibitive and human-derived matrices available on the market lack breast-specific indication and conformation (21,25). Disparate attempts at off-label constructs have been reported with AlloDerm® matrix to achieve easier ADM coverage for pre-pectoral placement. Whenever seeking complete coverage, only partial wrapping can often be achieved, especially when larger expanders are used (21,25).
The heterogeneity of literature data concerning two-stage ADM-assisted pre-pectoral reconstruction may reflect non-standardised implant wrapping procedures which lead to centre-to-centre variability. Our early experience with a specific standardized ADM wrapping technique for complete TE coverage reveals 12.5% total complications, and only 3.1% failure rate, fully in line with good clinical practice found in the literature so far (40,41). Our results are also in line with those reported in recent BRAXON®Fast publications (DTI procedures) (18,26,42). Within a standard patient selection, pre-pectoral TE placement with complete dermal coverage proves feasible with successful early clinical outcomes. A 0.6-mm thick and preshaped ADM easily conforms to the expander profile and histological analyses suggest a proper dynamic integration of the scaffold across the expanding process.
Understanding the cellular and molecular mechanisms behind ADM integration is crucial for improving surgical techniques and results in breast reconstruction. Our analyses confirmed matrix integration with cells and vascularization, revealing a thin cellular lining similar to the synovial membrane. Synovial metaplasia, likely stimulated by the mechanical stress of implants, is an adaptation mechanism to reduce friction between moving surfaces (23,42,43). It has been widely documented in capsules formed around silicone implants and it is indicative of a benign capsule (44). In fact, its presence is associated with Baker grade I and II capsules while its absence is typical of Baker grades III and IV capsules, possibly linking this formation with a protective effect against capsular contracture (43,45,46). Synovial metaplasia was observed to form also with other ADMs. Our histological results were similar to those reported in literature, with tissue biopsies showing blood vessels located just below the synovial metaplasia and good tissue integration overall with no signs of foreign body response (6,12). Our unit has experience with breast reconstruction performed using a titanium-coated polypropylene mesh and capsular tissue biopsies were also taken (47-49). A synovial metaplasia was observed, however, the presence of foreign body giant cells, marker of inflammation, indicates a different type of peri-implant tissue (internal data, not shown). Similarly, inflammation in such tissue was also confirmed in one previous work of ours (50). In fact, the inflammatory response initiated with the foreign body reaction can be either exacerbated or prolonged by the presence of a synthetic material, which ultimately leads to unregulated and continued stimulation of fibrosis with increased risk of capsular contracture (44,51). Hence, once again there is confirmation that, by promoting modulation of inflammation, the ADM as implant coverage creates a vascularised benign capsule integrated into the surrounding tissue that exerts a protective effect against capsular contracture (4,16).
This study is not without limitations. The retrospective and single-institution framework inevitably comes with potential surgical bias. Additionally, the small patient cohort as well as early follow-up do not allow for long-term conclusions to be drawn. Noteworthy issues such as postoperative pain and analgesic requirements, aesthetic outcomes and patient-reported outcomes were not evaluated. They will be subjects of our follow-up work, also including more extensive histopathological qualitative and quantitative analyses.
Acellular dermal matrices have revolutionized PPBR, providing surgeons with a powerful tool to enhance implant support, reduce complications, and improve overall outcomes. With this work, we have proved that a device born for DTI PPBR can be safely and effectively used in two-stage PPBR as it follows tissue expansion and successfully integrates in the surroundings. Even if this piece added to the puzzle that is Braxon body of literature, surgeons’ knowledge on ADM in breast reconstruction has increased and, ultimately, patients can be offered the most appropriate reconstructive technique, tailored on their characteristics and needs. Additionally, despite the two-stage procedure is recognised as least cost-effective and there are reports of patients’ lower quality of life (QoL) compared to DTI procedures (52), by placing the expander in the pre-pectoral position the functional and aesthetic benefits of the muscle-sparing technique are maintained. Cost-benefit analyses do not consider the cost of the pectoralis muscle loss and its fallout on patients’ QoL. We believe two-stage pre-pectoral reconstruction to be the best alternative to submuscular breast reconstruction while being at the same time the best compromise for patients non-ideal for a pre-pectoral DTI procedure.
Despite challenges, ongoing research and refinements aim to boost ADMs benefits in breast reconstruction, solidifying their role in modern surgical approaches. Investigating ADM-host tissue interactions and considering factors like processing techniques, patient characteristics, and medical conditions will refine and expand their applications.
Conclusions
The placement of ADM-covered TEs in pre-pectoral position combines the excellent aesthetic and functional results of the pre-pectoral philosophy with a quite safer and more prudent two-step approach. Our experience is one of the first with this technique and BRAXON®Fast and has shown encouraging results: the ADM successfully integrates in the dynamic environment created during tissue expansion and becomes a vascularised new self-tissue. Complications rates are low and such ADM-assisted two-stage pre-pectoral reconstructive technique is a safe, practical, and reproducible method.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-432/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-432/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-432/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-432/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Azienda Ospedaliero Universitaria Careggi (No. #16.069_AOUC) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Di Micco R, Santurro L, Lapiana G, et al. Pre-pectoral implant-based breast reconstruction after mastectomy: a narrative review. Ann Breast Surg 2023;7:27.
- Masià J. iBAG Working Group. The largest multicentre data collection on prepectoral breast reconstruction: The iBAG study. J Surg Oncol 2020;122:848-60. [Crossref] [PubMed]
- Bassetto F, Pandis L, Facchin F, et al. Braxon(®)-assisted prepectoral breast reconstruction: A decade later. Front Surg 2022;9:1009356. [Crossref] [PubMed]
- Cramer MC, Badylak SF. Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior. Ann Biomed Eng 2020;48:2132-53. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon(®) acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Onesti MG, Maruccia M, Di Taranto G, et al. Clinical, histological, and ultrasound follow-up of breast reconstruction with one-stage muscle-sparing "wrap" technique: A single-center experience. J Plast Reconstr Aesthet Surg 2017;70:1527-36. [Crossref] [PubMed]
- Iqbal FM, Bhatnagar A, Vidya R. Host Integration of an Acellular Dermal Matrix: Braxon Mesh in Breast Reconstruction. Clin Breast Cancer 2016;16:e209-11. [Crossref] [PubMed]
- Caputo GG, Franchini Z, Maritan M, et al. Daily serum collection after acellular dermal matrix-assisted breast reconstruction. Arch Plast Surg 2015;42:321-6. [Crossref] [PubMed]
- Bush K, Gertzman AA. Process Development and Manufacturing of Human and Animal Acellular Dermal Matrices. In: Albanna MZ, Holmes JH IV. editors. Skin Tissue Engineering and Regenerative Medicine. Amsterdam, NL: Elsevier; 2016:83-108.
- Katiyar S, Singh D, Kumari S, et al. Novel strategies for designing regenerative skin products for accelerated wound healing. 3 Biotech 2022;12:316.
- Zheng M, Wang X, Chen Y, et al. A Review of Recent Progress on Collagen-Based Biomaterials. Adv Healthc Mater 2023;12:e2202042. [Crossref] [PubMed]
- Boháč M, Danišovič Ľ, Koller J, et al. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur J Histochem 2018;62:2873. [Crossref] [PubMed]
- Keating JH, Melidone R, Garcia-Polite F. Preclinical Evaluation of Mesh Implants: The Pathologist's Perspective. Toxicol Pathol 2019;47:379-89. [Crossref] [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg 2010;126:1842-7. [Crossref] [PubMed]
- Berna G, Cawthorn SJ. Long term follow-up on prepectoral ADM-assisted breast reconstruction: evidences after 4 years. Eur J Plast Surg 2017;40:255-8.
- Polotto S, Pedrazzi G, Bergamini M, et al. ADM-Assisted Direct-to-Implant Prepectoral Breast Reconstruction in Postmastectomy Radiation Therapy Setting: Long-Term Results. Clin Breast Cancer 2023;23:704-11. [Crossref] [PubMed]
- Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77-86. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631. [Crossref] [PubMed]
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Boháč M, Danišovič Ľ, Danihel Ľ, et al. Histological and immunohistochemical characteristics of capsular synovial metaplasias that form around silicone breast implants. Biologia 2018;73:107-12.
- Chopra K, Buckingham B, Matthews J, et al. Acellular dermal matrix reduces capsule formation in two-stage breast reconstruction. Int Wound J 2017;14:414-9. [Crossref] [PubMed]
- Woo A, Harless C, Jacobson SR. Revisiting an Old Place: Single-Surgeon Experience on Post-Mastectomy Subcutaneous Implant-Based Breast Reconstruction. Breast J 2017;23:545-53. [Crossref] [PubMed]
- Berna G, De Grazia A, Antoniazzi E, et al. Novel three-dimensional acellular dermal matrix for prepectoral breast reconstruction: First year in review with BRAXON®Fast. Front Surg 2022;9:970053. [Crossref] [PubMed]
- Quintero Sierra LA, Busato A, Zingaretti N, et al. Tissue-Material Integration and Biostimulation Study of Collagen Acellular Matrices. Tissue Eng Regen Med 2022;19:477-90. [Crossref] [PubMed]
- Elswick SM, Harless CA, Bishop SN, et al. Prepectoral Implant-Based Breast Reconstruction with Postmastectomy Radiation Therapy. Plast Reconstr Surg 2018;142:1-12. [Crossref] [PubMed]
- Sigalove S. Prepectoral breast reconstruction and radiotherapy-a closer look. Gland Surg 2019;8:67-74. [Crossref] [PubMed]
- Sbitany H, Gomez-Sanchez C, Piper M, et al. Prepectoral Breast Reconstruction in the Setting of Postmastectomy Radiation Therapy: An Assessment of Clinical Outcomes and Benefits. Plast Reconstr Surg 2019;143:10-20. [Crossref] [PubMed]
- Banuelos J, Abu-Ghname A, Vyas K, et al. Should Obesity Be Considered a Contraindication for Prepectoral Breast Reconstruction? Plast Reconstr Surg 2020;145:619-27. [Crossref] [PubMed]
- Major MR, Wong VW, Nelson ER, et al. The foreign body response: at the interface of surgery and bioengineering. Plast Reconstr Surg 2015;135:1489-98. [Crossref] [PubMed]
- Slade CL. Subcutaneous mastectomy: acute complications and long-term follow-up. Plast Reconstr Surg 1984;73:84-90.
- Chopra K, Singh D, Hricz N, et al. Two-stage Prosthetic Prepectoral Breast Reconstruction: Comparing Tissue Expansion with Carbon Dioxide and Saline. Plast Reconstr Surg Glob Open 2019;7:e2051. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Staged Suprapectoral Expander/Implant Reconstruction without Acellular Dermal Matrix following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;139:30-9. [Crossref] [PubMed]
- Hammond DC, Schmitt WP, O'Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]
- Schaeffer CV, Dassoulas KR, Thuman J, et al. Early Functional Outcomes After Prepectoral Breast Reconstruction: A Case-Matched Cohort Study. Ann Plast Surg 2019;82:S399-403. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Tellarini A, Garutti L, Corno M, et al. Immediate post-mastectomy prepectoral breast reconstruction with animal derived acellular dermal matrices: A systematic review. J Plast Reconstr Aesthet Surg 2023;86:94-108. [Crossref] [PubMed]
- Knight HJ, Musgrove JJ, Youssef MMG, et al. Significantly reducing implant loss rates in immediate implant-based breast reconstruction: A protocol and completed audit of quality assurance. J Plast Reconstr Aesthet Surg 2020;73:1043-9. [Crossref] [PubMed]
- Vidya R, Berna G, Sbitany H, et al. Prepectoral implant-based breast reconstruction: a joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019;13:927. [Crossref] [PubMed]
- di Pompeo FS, Firmani G, Paolini G, et al. Immediate prepectoral breast reconstruction using an ADM with smooth round implants: A prospective observational cohort study. J Plast Reconstr Aesthet Surg 2023;80:56-65. [Crossref] [PubMed]
- Bassetto F, Scarpa C, Caccialanza E, et al. Histological features of periprosthetic mammary capsules: silicone vs. polyurethane. Aesthetic Plast Surg 2010;34:481-5. [Crossref] [PubMed]
- Gorgy A, Barone N, Nepon H, Dalfen J, Efanov JI, Vorstenbosch J. Implant-based breast surgery and capsular formation : when, how and why ?— a narrative review. 2023;0–2.
- Bui JM, Perry T, Ren CD, et al. Histological characterization of human breast implant capsules. Aesthetic Plast Surg 2015;39:306-15. Erratum in: Aesthetic Plast Surg 2015;39:316-7. [Crossref] [PubMed]
- Mohan AS, Sullivan J, Tenenbaum MM, et al. Toward a Consensus Aproach for Assessing Capsular Contracture Severity and Progression: A Systematic Review. Plast Reconstr Surg 2024;153:7-22. [Crossref] [PubMed]
- Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous Direct-to-Implant Breast Reconstruction: Surgical, Functional, and Aesthetic Results after Long-Term Follow-Up. Plast Reconstr Surg Glob Open 2015;3:e574. [Crossref] [PubMed]
- Bernini M, Meattini I, Saieva C, et al. Pre-pectoral breast reconstruction: early and long-term safety evaluation of 146 unselected cases of the early pre-pectoral era of a single-institution, including cases with previous breast irradiation and post-mastectomy radiation therapy. Breast Cancer 2022;29:302-13. [Crossref] [PubMed]
- Casella D, Calabrese C, Bianchi S, et al. Subcutaneous Tissue Expander Placement with Synthetic Titanium-Coated Mesh in Breast Reconstruction: Long-term Results. Plast Reconstr Surg Glob Open 2015;3:e577. [Crossref] [PubMed]
- Gentile P, Bernini M, Orzalesi L, et al. Titanium-coated polypropylene mesh as innovative bioactive material in conservatives mastectomies and pre-pectoral breast reconstruction. Bioact Mater 2021;6:4640-53. [Crossref] [PubMed]
- Bergmann PA, Becker B, Mauss KL, et al. Titanium-coated polypropylene mesh (TiLoop Bra®) - An effective prevention for capsular contracture? Eur J Plast Surg 2014;37:339-46.
- Cattelani L, Polotto S, Arcuri MF, et al. One-Step Prepectoral Breast Reconstruction With Dermal Matrix-Covered Implant Compared to Submuscular Implantation: Functional and Cost Evaluation. Clin Breast Cancer 2018;18:e703-11. [Crossref] [PubMed]


