
Analysis of risk factors for lateral lymph node metastasis in T1 stage papillary thyroid carcinoma: a retrospective cohort study
Highlight box
Key findings
• In this retrospective study of 3,332 patients with T1 stage papillary thyroid carcinoma (PTC), we found factors (male gender, upper lobe tumor, larger volume, and multifocality) linked to lateral lymph node metastasis (LLNM) risk. Patients without these risks, particularly in T1b stage PTC, may benefit from short-term active surveillance (AS), underscoring the importance of an assertive approach in cases with increased tumor size.
What is known and what is new?
• In early-stage PTC, there is a propensity for cervical lymph node metastasis, with lateral compartment involvement correlating with an adverse prognosis for patients.
• In our study, tumor volume, not diameter, strongly correlated with LLNM risk. Notably, younger patients showed no significant increase in this risk. In T1a stage PTC, males had a closer association with LLNM, while in T1b stage PTC, tumor size played a more crucial role.
What is the implication, and what should change now?
• In clinical practice, patients with identified risk factors necessitate routine follow-up and careful consideration of the optimal timing for surgical intervention due to heightened vulnerability to lateral nodal metastasis. Nevertheless, those lacking these risks, especially younger patients, may consider short-term AS, justifying its adoption when these risk factors are absent. In T1a stage PTC, male gender prompts careful evaluation for immediate surgical intervention. In T1b stage PTC, increased tumor size emphasizes the necessity for a more assertive treatment approach.
Introduction
Background
Papillary thyroid carcinoma (PTC) constitutes the most prevalent histological variant among thyroid malignancies, accounting for approximately 89.1% of all cases. This statistic, however, shows a marginal decline between 2014 and 2018 (1). Patients diagnosed with PTC typically demonstrate favorable prognostic outcomes and low mortality rates. Nonetheless, early-stage metastasis to the cervical lymph nodes is not uncommon. Prior research (2-5) indicates that lymph node metastases are present in about 20% to 90% of PTC cases. Although central lymph node metastasis does not markedly alter the prognosis for PTC patients (6), the emergence of lateral lymph node metastasis (LLNM, N1b) often necessitates more complex and prolonged surgical procedures, potentially impacting patient prognosis adversely (7,8).
A study by Sapuppo et al. (7) categorized PTC patients based on their postoperative pathologic N status. Findings indicated that individuals classified at the N1b stage showed an increased incidence of structural diseases, including locoregional lymph node and/or distant metastases, compared to those in the N0 and N1a stages. At their final follow-up, N1b stage patients exhibited a higher likelihood of persistent or recurrent disease relative to those in the N1a category. Moreover, those with lateral LN metastasis demonstrated reduced disease-free and 10-year disease-related survival rates (9,10). A notable study observed a 3.0% mortality rate among N1b patients, significantly higher than that in N1a and N0 patients (11), suggesting that LLN positivity is a strong prognostic indicator for poor outcomes in PTC.
The American Thyroid Association (ATA) management guidelines for differentiated thyroid cancer (DTC) recommend central and/or lateral lymph node dissection when metastasis is clinically or radiographically evident, while cautioning against routine prophylactic dissection of lateral lymph nodes (12). The efficacy of prophylactic level VI (central) neck dissection in cN0 disease remains a topic of debate (12). Confirmed LLNMs necessitate additional lateral lymph node dissection, extending the surgery’s complexity and duration. Such procedures also increase the likelihood of postoperative complications, including celiac leakage, hemorrhage, nerve injury, shoulder discomfort, and restricted mobility (13). Consequently, the development of prognostic methods for LLNM is essential in managing node metastasis and recurrence in PTC.
Despite extensive research into LLNM risk factors in PTC, findings have been inconsistent (8,14-20). Our study explored major risk factors such as patient age, gender, primary tumor location, tumor diameter, multifocality, and bilaterality. Additionally, we hypothesized that primary tumor volume and duration of active surveillance (AS) post-diagnosis could also contribute to LLNM risk. These variables were thus comprehensively integrated into our analysis.
AS involves monitoring cancer patients without immediate surgical or radiation intervention unless the disease progresses. In 1993, Dr. Akira Miyauchi proposed delayed surgical intervention as an alternative to immediate surgery for papillary thyroid microcarcinoma (PTMC, tumor diameter <1 cm) at a symposium hosted at Kuma Hospital, Japan. Subsequent trials in 2003 and 2010 corroborated the feasibility of this approach (21,22), and numerous patients in Korea have been studied (23), showcasing AS as a promising alternative for PTMC treatment. Ho et al.’s comprehensive study (24) found no significant mortality risk difference between 1.0- and 2.0-cm thyroid tumors. However, a tumor diameter of >2 cm independently correlates with an increased risk of cancer-related death. Therefore, for all T1 stage (<2 cm) tumors, AS may be a feasible alternative to immediate surgery (24,25). AS also appears as a potential therapeutic option for recurrent lymph node metastasis in DTC (26,27). During AS, most low-risk PTC patients did not develop new lymph node metastases (28-30). However, the direct link between AS and the occurrence of LLN metastasis has not been comprehensively documented.
Objective
This retrospective cohort study aims to identify risk factors for LLNM development, providing insights for the timing of surgical interventions in clinical practice and contributing to informed clinical decision-making and patient welfare. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-470/rc).
Methods
Patients
This study entailed a retrospective cohort analysis of 3,332 patients diagnosed with PTC, who underwent initial thyroidectomy at West China Hospital, Sichuan University, from June 2017 to February 2023. A detailed methodology flowchart is provided in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of West China Hospital of Sichuan University (2023 No. 2098). Individual consent for this retrospective analysis was waived. All participants underwent their first thyroid surgery and were confirmed to have no other histopathological types of thyroid carcinoma. The cohort was bifurcated based on the presence or absence of LLNM, as ascertained by postoperative pathological evaluation. Further, these patients were segregated into T1a (<10 mm) and T1b (>10 mm) categories, contingent on the maximum diameter of the tumor.
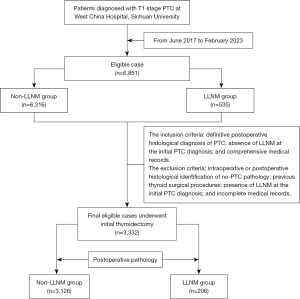
The inclusion criteria encompassed: definitive postoperative histological diagnosis of PTC; absence of suspicious LLNM on ultrasonography at the initial PTC diagnosis; and comprehensive medical records. The exclusion criteria included: intraoperative or postoperative histopathological identification of non-PTC pathology; previous thyroid surgical procedures; presence of LLNM on ultrasonography or distant metastasis on computed tomography/magnetic resonance imaging (CT/MRI) at the initial PTC diagnosis; and incomplete medical records.
Data collection
Clinical and pathological data were collated from electronic medical records and pathology reports.
Patient demographic information (gender and age), and the time of initial fine-needle aspiration biopsy (FNAB) confirming PTC can be retrieved from the electronic medical records. The ultrasonography report provided tumor characteristics (maximum diameter, volume, location) and clinical details (multifocality, bilaterality, and LLNM status). The tumor volume (in mm3) was computed employing the ellipsoid volume equation: π/6 × length × width × height. The features of suspect malignant lymph node involvement include (with at least one of the following features): (I) microcalcifications; (II) partially cystic appearance; (III) increased peripheral or diffuse vascularity; (IV) hyperechoic tissue looking like thyroid. The characteristic of indeterminate lymph node: disappearance of lymphatic hilum and at least one of the following characteristics: round shape; increased short axis, ≥8 mm in level II and ≥5 mm in levels III and IV; absence of central vascularization (12,31,32). We conducted lymph node assessment according to those criteria. For a few indeterminate lymph nodes, after discussing with the patient, we opted for either lymph node fine-needle aspiration with thyroglobulin washout fluid testing or immediate surgery. For the LLNM group, the period of AS before surgery was demarcated as the interval from the initial FNAB confirming PTC, to the first detection of LLNM via preoperative ultrasound, subsequently corroborated by postoperative pathology. For the non-LLNM cohort, this duration was defined as the time span between the initial FNAB diagnosis of PTC and the admission for surgery. Quantitative data, including age, primary tumor’s maximum diameter and volume, and waiting time for surgery, were transformed into qualitative categories based on predetermined cut-off values.
Statistical analysis
Continuous variables were converted into categorical data for analysis using SPSS version 25. The Chi-squared test was employed to compare demographic and tumor characteristics, including gender, age, tumor size, tumor location, and AS duration between the LLNM and non-LLNM groups. Multifactorial analysis was performed using binary logistic regression. A P value of less than 0.05 (two-tailed) was considered indicative of statistical significance.
Results
Patient characteristics and group analysis
Among 3,332 PTC patients meeting inclusion and exclusion criteria, 206 presented with LLNM. The clinical and pathological characteristics of all participants are delineated in Table 1. The average AS time of the LLNM group is 137.9±145.7 days. The LLNM group had a significantly higher proportion of males at 37.4% (77/206) compared to the non-LLNM group at 23.5% (P<0.001). The mean age in the metastasis cohort was 41.6±10.9 years, which did not significantly differ from that of the non-metastasis group (P=0.729). Tumor location was categorized as upper lobe or non-upper lobe (inclusive of middle and lower lobes and the isthmus). A higher proportion of upper lobe tumors was observed in the LLNM group (38.8%) compared to the non-LLNM group (25.0%) (P<0.001). Additionally, significant differences were noted in the maximum tumor diameter (11.5±4.1 mm in the LLNM group versus 9.0±3.5 mm in the non-LLNM group, P<0.001) and maximum tumor volume (603.1±569.4 mm3 in the LLNM group versus 318.6±377.2 mm3 in the non-LLNM group, P<0.001). Higher incidences of multifocal and bilateral tumors were observed in the LLNM group (29.1% and 18.0%, respectively) compared to the non-LLNM group (P<0.05 for both).
Table 1
| Category | Non-LLNM (n=3,126) | LLNM (n=206) | Total | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Sex | 1.945 (1.450–2.610) | <0.001 | |||
| Female | 2,392 (76.5) | 129 (62.6) | 2,521 (75.7) | ||
| Male | 734 (23.5) | 77 (37.4) | 811 (24.3) | ||
| Age (years) | 0.939 (0.595–1.482) | 0.788 | |||
| ≤55 | 2,773 (88.7) | 184 (89.3) | 2,957 (88.7) | ||
| >55 | 353 (11.3) | 22 (10.7) | 375 (11.3) | ||
| Mean ± SD | 41.3±10.8 | 41.6±10.9 | 0.729 | ||
| Tumor location | 0.525 (0.393–0.703) | <0.001 | |||
| Upper lobe | 782 (25.0) | 80 (38.8) | 862 (25.9) | ||
| Non-upper lobe | 2,344 (75.0) | 126 (61.2) | 2,470 (74.1) | ||
| Tumor volume (mm3) | <0.001 | ||||
| <319 | 2,149 (68.7) | 78 (37.9) | 2,227 (66.8) | 1.000 | |
| 319–603 | 504 (16.1) | 50 (24.3) | 554 (16.6) | 2.733 (1.892–3.949) | <0.001 |
| >603 | 473 (15.1) | 78 (37.9) | 551 (16.5) | 4.543 (3.269–6.315) | <0.001 |
| Mean ± SD | 318.6±377.2 | 603.1±569.4 | <0.001 | ||
| Tumor diameter (mm) | 2.917 (2.167–3.927) | <0.001 | |||
| ≤12 | 2,595 (83.0) | 129 (62.6) | 2,724 (81.8) | ||
| >12 | 531 (17.0) | 77 (37.4) | 608 (18.2) | ||
| Mean ± SD | 9.0±3.5 | 11.5±4.1 | <0.001 | ||
| Multifocality | 2.444 (1.780–3.354) | <0.001 | |||
| No | 2,676 (85.6) | 146 (70.9) | 2,822 (84.7) | ||
| Yes | 450 (14.4) | 60 (29.1) | 510 (15.3) | ||
| Bilaterality | 1.907 (1.312–2.771) | 0.001 | |||
| No | 2,804 (89.7) | 169 (82.0) | 2,973 (89.2) | ||
| Yes | 322 (10.3) | 37 (18.0) | 359 (10.8) | ||
| AS time (months) | 0.983 (0.719–1.381) | 0.978 | |||
| ≤6 | 2,350 (75.2) | 155 (75.2) | 2,505 (75.2) | ||
| >6 | 776 (24.8) | 51 (24.8) | 827 (24.8) |
Data are reported as n (%), unless noted otherwise. P values represent the statistically difference between the groups with and without LLNMs, unless noted otherwise. LLNM, lateral lymph node metastasis; PTC, papillary thyroid carcinoma; OR, odds ratio; CI, confidence interval; SD, standard deviation; AS, active surveillance.
Univariate and multivariate analyses
Univariate analysis (Table 1) revealed that male gender, primary tumor location in the upper lobe, larger tumor volume, greater tumor diameter, multifocal, and bilateral tumors were all significantly associated with LLNM (all P<0.001). The multivariate analysis was presented in Table 2. Male patients exhibited approximately double the risk of LLNM compared to females [odds ratio (OR) =1.782, P<0.001]. The presence of primary tumors in the upper lobes (OR =1.975, P<0.001), larger tumor volumes (319–603 mm3, OR =2.546, P<0.001; >603 mm3, OR =4.784, P<0.001), and multifocality (OR =3.254, P<0.001) were also significantly correlated with an increased risk of LLNM. Gender-specific analyses (Table 3) did not reveal a significant correlation between AS duration and LLNM risk.
Table 2
| Variables | OR (95% CI) | P value |
|---|---|---|
| Sex | ||
| Female | Reference | |
| Male | 1.782 (1.315–2.417) | <0.001 |
| Age (years) | ||
| ≤55 | 1.207 (0.755–1.931) | 0.432 |
| >55 | Reference | |
| Tumor location | ||
| Upper lobe | 1.975 (1.461–2.670) | <0.001 |
| Non-upper lobe | Reference | |
| Tumor volume (mm3) | <0.001 | |
| <319 | Reference | |
| 319–603 | 2.546 (1.731–3.744) | <0.001 |
| >603 | 4.784 (2.676–8.553) | <0.001 |
| Tumor diameter (mm) | 0.359 | |
| ≤12 | Reference | |
| >12 | 0.900 (0.525–1.546) | 0.704 |
| Multifocality | 3.254 (1.976–5.358) | <0.001 |
| Bilaterality | 0.606 (0.337–1.089) | 0.094 |
| AS time (months) | ||
| ≤6 | Reference | |
| >6 | 1.080 (0.771–1.514) | 0.654 |
LLNM, lateral lymph node metastasis; PTC, papillary thyroid carcinoma; OR, odds ratio; CI, confidence interval; AS, active surveillance.
Table 3
| AS time (months) | Non-LLNM | LLNM | Total | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Female (n=2,521) | |||||
| ≤6 | 1,789 | 95 | 1,884 | 1.062 (0.710–1.588) | 0.770 |
| >6 | 603 | 34 | 637 | ||
| ≤12 | 2,131 | 119 | 2,250 | 0.686 (0.355–1.325) | 0.259 |
| >12 | 261 | 10 | 271 | ||
| ≤24 | 2,325 | 129 | 2,454 | – | 0.100 |
| >24 | 67 | 0 | 67 | ||
| Male (n=811) | |||||
| ≤6 | 561 | 60 | 621 | 0.919 (0.522–1.616) | 0.769 |
| >6 | 173 | 17 | 190 | ||
| ≤12 | 647 | 71 | 718 | 0.628 (0.265–1.489) | 0.287 |
| >12 | 87 | 6 | 93 | ||
| ≤24 | 719 | 74 | 793 | 1.943 (0.550–6.868) | 0.520 |
| >24 | 15 | 3 | 18 |
PTC, papillary thyroid carcinoma; AS, active surveillance; LLNM, lateral lymph node metastasis; OR, odds ratio; CI, confidence interval.
Subgroup analysis: T1a and T1b groups
Supplementary material present clinical and pathological data for T1a and T1b subgroups, respectively. Univariate analysis (Tables S1,S2) indicated common LLNM risk factors: male gender, upper lobe tumor location, larger tumor size, and multifocality. Multifactorial regression analysis (Table 4) highlighted that gender was a significant risk factor for LLNM in the T1a group (P<0.001) but not in the T1b group (P=0.097). Furthermore, no significant differences were observed in age and AS duration between LLNM and non-LLNM groups in both subgroups (Tables S3,S4).
Table 4
| Variables | T1a (n=2,318) | T1b (n=1,014) | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Sex | |||||
| Female | Reference | Reference | |||
| Male | 2.253 (1.444–3.515) | <0.001 | 1.426 (0.937–2.169) | 0.097 | |
| Age (years) | |||||
| ≤55 | 0.712 (0.392–1.295) | 0.266 | 2.075 (0.968–4.448) | 0.061 | |
| >55 | Reference | Reference | |||
| Tumor location | |||||
| Upper lobe | 2.131 (1.367–3.323) | 0.001 | 1.951 (1.286–2.960) | 0.002 | |
| Non-upper lobe | Reference | Reference | |||
| Tumor volume (mm3) | |||||
| ≤140 | Reference | Reference | |||
| >140 | 1.960 (0.884–4.346) | 0.098 | 2.072 (1.222–3.516) | 0.007 | |
| Tumor diameter (mm) | |||||
| ≤7 | Reference | Reference | |||
| >7 | 0.955 (0.432–2.111) | 0.909 | 1.268 (0.745–2.158) | 0.382 | |
| Multifocality | 2.780 (1.273–6.072) | 0.01 | 3.461 (1.747–6.857) | <0.001 | |
| Bilaterality | 0.791 (0.321–1.950) | 0.611 | 0.596 (0.268–1.326) | 0.205 | |
| AS time (months) | |||||
| ≤6 | Reference | Reference | |||
| >6 | 1.314 (0.821–2.103) | 0.256 | 0.944 (0.578–1.541) | 0.817 | |
LLNM, lateral lymph node metastasis; PTC, papillary thyroid carcinoma; OR, odds ratio; CI, confidence interval; AS, active surveillance.
Discussion
In this extensive retrospective analysis of 3,332 patients, we observed a positive association between factors such as male gender, upper lobe tumor location, larger tumor volume, and multifocality with the risk of LLNM. This finding underscores the necessity of routine follow-up and careful consideration of the optimal timing for surgical intervention, particularly when these factors coexist. Conversely, factors like age, tumor diameter, bilateral tumor presence, and extended AS duration did not demonstrate a substantial correlation with lateral nodal involvement.
Aligning with Mao et al.’s meta-analysis (14), our study reaffirms male sex as a risk factor for LLNM in T1 stage PTC patients, highlighting a higher propensity for LLNM in men. This gender-based disparity in LLNM incidence aligns with several studies (15,33), although it remains a subject of debate in other research (34,35). Notably, the adverse prognosis associated with PTC tends to be more pronounced in men, despite its higher prevalence in women (36). This suggests the need for rigorous evaluation of immediate thyroid surgery in male patients, particularly in T1a stage PTC.
Though the traditional belief that younger age (<55 years) is a risk factor for lymph node metastasis (17,34), our study found no significant age-related differences in LLNM risk, potentially supporting the potential role of AS in specific cases.
Primary tumor location significantly affects lymph node dissemination, as supported by our findings in agreement with prior research (19,37-39). Given the complex drainage patterns and higher postoperative complication risks associated with upper thyroid tumors, accurate LLNM assessment in clinical practice is paramount.
While PTMC is generally considered low-risk for LLNM (12), our study suggests that larger tumor diameters do not necessarily predict LLNM, diverging from some previous research (40). By incorporating tumor volume in our analysis, we found a strong association between greater tumor volumes and an increased risk of LLNM. This finding is supported by research emphasizing tumor volume as a more reliable prognostic marker than diameter (41). These results highlight the importance of careful surgical planning for patients with larger tumor volumes.
Our analysis identified multifocality as a LLNM risk factor, contrary to some studies that suggest both multifocality and bilaterality increase LLNM risk (39). Multifocality’s prognostic significance, especially in tumors larger than 1 cm, is well-established (42). However, our findings do not support the hypothesis that bilaterality, an indicator of tumor invasiveness, heightens LLNM risk.
Our study delves into the potential impact of AS duration on LLNM risk. With the emerging role of AS in managing T1a and potentially T1b stage PTC (25,43), there is increasing focus on understanding the association between surveillance duration and LLNM risk. Within Table 3, we performed separate analyses employing surveillance duration thresholds of 6, 12, and 24 months (Table 3, all P values >0.05). The selection of these thresholds values was informed by clinical practice. These analyses reveal that, for those with stage T1 PTC, there were no notable differences in the distribution of time to AS between the LLNM and non-LLNM groups. In the subgroups of T1a and T1b, we encountered equivalent results. Hence, our findings suggest that short-term AS (≤24 months) may not considerably increase the risk of LLNM in T1 stage PTC patients, which is in accordance with the conclusions of previous studies (44,45). Further research, particularly focusing on long-term surveillance, is warranted to substantiate these findings.
LLNM is associated with a poor prognosis in patients (46). Nevertheless, the current indication for performing cervical lateral lymph node dissection in PTC cases remain subject to debate. According to the ATA management guidelines, patients are recommended for LLN dissection when clinical or radiographic evidence supports the presence of lymph node disease (12). Ultrasonography is the primary tool for diagnosing cN1b, but its sensitivity was found to be only 0.70 (95% CI: 0.68–0.72; I2=96.7%) (47). Building on our earlier discussion, preoperative patients presenting with male gender, upper lobe tumor location, larger tumor dimension, and multifocality are indicative of a heightened risk of LLNM. These high-risk factors discourage AS and instead favor lateral lymph node dissection surgery. The identification of high-risk factors for predicting LLN metastasis contributes to decisions regarding total thyroidectomy and lateral lymph node dissection in PTC patients, as well as guides surgeons in evaluating and treating cervical lateral lymph nodes during postoperative follow-up. Postoperative adjuvant therapy for PTC typically comprises TSH suppression therapy and radioactive iodine treatment (48). For high-risk thyroid cancer patients, TSH levels are generally maintained below 0.1 mU/L (12). Moreover, patients with suspected or confirmed lymph node metastasis and extrathyroidal tumor extension might necessitate an increased radioactive iodine dosage to further diminish the risk of recurrence (49). Consequently, for postoperative patients meeting all the aforementioned risk factors, it seems justifiable to adopt a proactive approach in deciding on TSH suppression therapy and radioactive iodine treatment to minimize the risk of PTC recurrence after surgery.
This study, being retrospective and single-center, has its limitations, including the lack of uniformity in tumor location assessment and the absence of a separate analysis for different types of lymph node metastasis. Future multicenter, prospective studies with larger sample sizes and extended follow-up periods are necessary to address these gaps and further explore the nuances of LLNM in PTC.
Conclusions
In summary, this retrospective analysis has identified several critical risk factors for LLNM in patients with T1 stage PTC. These include male gender, tumor location in the upper third of the thyroid gland, a maximum tumor volume exceeding 603 mm3, and the presence of multifocal tumors. In clinical practice, patients exhibiting this constellation of risk factors should give serious thought to surgical intervention due to their increased vulnerability to lateral nodal metastasis.
On the other hand, for patients not displaying these risk factors, the consideration of a short-term AS strategy may be appropriate. Our findings indicate that extended periods of AS, especially in younger patients, do not significantly escalate the risk of LLNM. This observation offers a viable justification for adopting AS in certain patient cohorts, particularly when the risk factors mentioned above are absent.
It is also noteworthy that in patients with T1a stage PTC, male gender should trigger a careful evaluation of the need for immediate surgical intervention. However, in the context of T1b stage PTC, the influence of gender appears less pronounced. Rather, an increase in tumor size emerges as a pivotal factor in elevating the risk of LLNM, underscoring the necessity for a more assertive treatment approach in these cases.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-470/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-470/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-470/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-470/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of West China Hospital of Sichuan University (2023 No. 2098). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Megwalu UC, Moon PK. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000-2018. Thyroid 2022;32:560-70. [Crossref] [PubMed]
- Wang Y, Zheng J, Hu X, et al. A retrospective study of papillary thyroid carcinoma: Hashimoto's thyroiditis as a protective biomarker for lymph node metastasis. Eur J Surg Oncol 2023;49:560-7. [Crossref] [PubMed]
- Luo Y, Zhao Y, Chen K, et al. Clinical analysis of cervical lymph node metastasis risk factors in patients with papillary thyroid microcarcinoma. J Endocrinol Invest 2019;42:227-36. [Crossref] [PubMed]
- Feng JW, Yang XH, Wu BQ, et al. Predictive factors for central lymph node and lateral cervical lymph node metastases in papillary thyroid carcinoma. Clin Transl Oncol 2019;21:1482-91. [Crossref] [PubMed]
- Wang Y, Deng C, Shu X, et al. Risk Factors and a Prediction Model of Lateral Lymph Node Metastasis in CN0 Papillary Thyroid Carcinoma Patients With 1-2 Central Lymph Node Metastases. Front Endocrinol (Lausanne) 2021;12:716728. [Crossref] [PubMed]
- Viola D, Materazzi G, Valerio L, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab 2015;100:1316-24. [Crossref] [PubMed]
- Sapuppo G, Palermo F, Russo M, et al. Latero-cervical lymph node metastases (N1b) represent an additional risk factor for papillary thyroid cancer outcome. J Endocrinol Invest 2017;40:1355-63. [Crossref] [PubMed]
- Huang Y, Yin Y, Zhou W. Risk Factors for Central and Lateral Lymph Node Metastases in Patients With Papillary Thyroid Micro-Carcinoma: Retrospective Analysis on 484 Cases. Front Endocrinol (Lausanne) 2021;12:640565. [Crossref] [PubMed]
- Ito Y, Miyauchi A. Prognostic factors and therapeutic strategies for differentiated carcinomas of the thyroid. Endocr J 2009;56:177-92.
- Moreno MA, Agarwal G, de Luna R, et al. Preoperative lateral neck ultrasonography as a long-term outcome predictor in papillary thyroid cancer. Arch Otolaryngol Head Neck Surg 2011;137:157-62. [Crossref] [PubMed]
- Sapuppo G, Tavarelli M, Russo M, et al. Lymph node location is a risk factor for papillary thyroid cancer-related death. J Endocrinol Invest 2018;41:1349-53. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Heng Y, Yang Z, Zhou L, et al. Risk stratification for lateral involvement in papillary thyroid carcinoma patients with central lymph node metastasis. Endocrine 2020;68:320-8. [Crossref] [PubMed]
- Mao J, Zhang Q, Zhang H, et al. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:265. [Crossref] [PubMed]
- Zhu J, Huang R, Yu P, et al. Male Gender Is Associated with Lymph Node Metastasis but Not with Recurrence in Papillary Thyroid Carcinoma. Int J Endocrinol 2022;2022:3534783. [Crossref] [PubMed]
- Kruijff S, Petersen JF, Chen P, et al. Patterns of structural recurrence in papillary thyroid cancer. World J Surg 2014;38:653-9. [Crossref] [PubMed]
- Zhao H, Huang T, Li H. Risk factors for skip metastasis and lateral lymph node metastasis of papillary thyroid cancer. Surgery 2019;166:55-60. [Crossref] [PubMed]
- Feng JW, Wu WX, Qi GF, et al. Nomograms based on sonographic and clinicopathological characteristics to predict lateral lymph node metastasis in classic papillary thyroid carcinoma. J Endocrinol Invest 2022;45:2043-57. [Crossref] [PubMed]
- Heng Y, Feng S, Yang Z, et al. Features of Lymph Node Metastasis and Structural Recurrence in Papillary Thyroid Carcinoma Located in the Upper Portion of the Thyroid: A Retrospective Cohort Study. Front Endocrinol (Lausanne) 2021;12:793997. [Crossref] [PubMed]
- Feng JW, Qin AC, Ye J, et al. Predictive Factors for Lateral Lymph Node Metastasis and Skip Metastasis in Papillary Thyroid Carcinoma. Endocr Pathol 2020;31:67-76. [Crossref] [PubMed]
- Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003;13:381-7. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34:28-35. [Crossref] [PubMed]
- Kwon H, Oh HS, Kim M, et al. Active Surveillance for Patients With Papillary Thyroid Microcarcinoma: A Single Center's Experience in Korea. J Clin Endocrinol Metab 2017;102:1917-25. [Crossref] [PubMed]
- Ho AS, Luu M, Zalt C, et al. Mortality Risk of Nonoperative Papillary Thyroid Carcinoma: A Corollary for Active Surveillance. Thyroid 2019;29:1409-17. [Crossref] [PubMed]
- Ho AS, Kim S, Zalt C, et al. Expanded Parameters in Active Surveillance for Low-risk Papillary Thyroid Carcinoma: A Nonrandomized Controlled Trial. JAMA Oncol 2022;8:1588-96. [Crossref] [PubMed]
- Walter LB, Scheffel RS, Zanella AB, et al. Active Surveillance of Differentiated Thyroid Cancer Metastatic Cervical Lymph Nodes: A Retrospective Single-Center Cohort Study. Thyroid 2023;33:312-20. [Crossref] [PubMed]
- Jerkovich F, Abelleira E, Bueno F, et al. Active Surveillance of Small Metastatic Lymph Nodes as an Alternative to Surgery in Selected Patients with Low-Risk Papillary Thyroid Cancer: A Retrospective Cohort Study. Thyroid 2022;32:1178-83. [Crossref] [PubMed]
- Ze Y, Zhang X, Shao F, et al. Active surveillance of low-risk papillary thyroid carcinoma: a promising strategy requiring additional evidence. J Cancer Res Clin Oncol 2019;145:2751-9. [Crossref] [PubMed]
- Sugitani I. Active surveillance of low-risk papillary thyroid microcarcinoma. Best Pract Res Clin Endocrinol Metab 2023;37:101630. [Crossref] [PubMed]
- Smulever A, Pitoia F. Conservative management of low-risk papillary thyroid carcinoma: a review of the active surveillance experience. Thyroid Res 2023;16:6. [Crossref] [PubMed]
- Ha EJ, Chung SR, Na DG, et al. 2021 Korean Thyroid Imaging Reporting and Data System and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2021;22:2094-123. [Crossref] [PubMed]
- Health Commission Of The People's Republic Of China N. National guidelines for diagnosis and treatment of thyroid cancer 2022 in China (English version). Chin J Cancer Res 2022;34:131-50. [Crossref] [PubMed]
- Ding J, Wu W, Fang J, et al. Male sex is associated with aggressive behaviour and poor prognosis in Chinese papillary thyroid carcinoma. Sci Rep 2020;10:4141. [Crossref] [PubMed]
- Liu Q, Pang WT, Dong YB, et al. Analysis of risk factors for lateral lymph node metastasis in papillary thyroid carcinoma: A retrospective cohort study. World J Otorhinolaryngol Head Neck Surg 2022;8:274-8. [Crossref] [PubMed]
- Masui T, Adachi S, Uemura H, et al. Risk factors for the lateral cervical lymph node metastasis of papillary thyroid carcinoma: A clinical study. Mol Clin Oncol 2023;18:25. [Crossref] [PubMed]
- Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol 2010;6:1771-9. [Crossref] [PubMed]
- Hunt JP, Buchmann LO, Wang L, et al. An analysis of factors predicting lateral cervical nodal metastases in papillary carcinoma of the thyroid. Arch Otolaryngol Head Neck Surg 2011;137:1141-5. [Crossref] [PubMed]
- Xiang D, Xie L, Xu Y, et al. Papillary thyroid microcarcinomas located at the middle part of the middle third of the thyroid gland correlates with the presence of neck metastasis. Surgery 2015;157:526-33. [Crossref] [PubMed]
- So YK, Kim MJ, Kim S, et al. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg 2018;50:94-103. [Crossref] [PubMed]
- Hu D, Zhou J, He W, et al. Risk factors of lateral lymph node metastasis in cN0 papillary thyroid carcinoma. World J Surg Oncol 2018;16:30. [Crossref] [PubMed]
- Lim ST, Jeon YW, Suh YJ. The Prognostic Values of Preoperative Tumor Volume and Tumor Diameter in T1N0 Papillary Thyroid Cancer. Cancer Res Treat 2017;49:890-7. [Crossref] [PubMed]
- Kim KJ, Kim SM, Lee YS, et al. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol 2015;22:125-31. [Crossref] [PubMed]
- Sakai T, Sugitani I, Ebina A, et al. Active Surveillance for T1bN0M0 Papillary Thyroid Carcinoma. Thyroid 2019;29:59-63. [Crossref] [PubMed]
- Miyauchi A, Ito Y, Fujishima M, et al. Long-Term Outcomes of Active Surveillance and Immediate Surgery for Adult Patients with Low-Risk Papillary Thyroid Microcarcinoma: 30-Year Experience. Thyroid 2023;33:817-25. [Crossref] [PubMed]
- Ito Y, Miyauchi A. Active Surveillance May Be the Best Initial Management for Papillary Thyroid Microcarcinoma. J Endocr Soc 2023;7:bvad063. [Crossref] [PubMed]
- Bayadsi H, Nylén C, Sandström M, et al. Risk factors for recurrent disease in small papillary thyroid cancers - a Swedish register-based study. Langenbecks Arch Surg 2023;408:162. [Crossref] [PubMed]
- Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: Diagnosis of central and lateral compartment nodal metastases. Eur J Radiol 2019;112:14-21. [Crossref] [PubMed]
- Houten PV, Netea-Maier RT, Smit JW. Differentiated thyroid carcinoma: An update. Best Pract Res Clin Endocrinol Metab 2023;37:101687. [Crossref] [PubMed]
- Chu KP, Baker S, Zenke J, et al. Low-Activity Radioactive Iodine Therapy for Thyroid Carcinomas Exhibiting Nodal Metastases and Extrathyroidal Extension May Lead to Early Disease Recurrence. Thyroid 2018;28:902-12. [Crossref] [PubMed]


