
Normative electromyography data and influencing factors in intraoperative neuromonitoring using adhesive skin electrodes during thyroid surgery
Highlight box
Key findings
• A larger surgical extent, obesity, male sex, and age >55 years showed significantly lower evoked amplitudes in thyroid intraoperative neuromonitoring using skin electrodes.
What is known and what is new?
• Skin electrodes have been reported to be a useful alternative recording method for intraoperative neuromonitoring.
• The evoked amplitude recorded with the skin electrodes was relatively low. A larger surgical extent, obesity, male sex, and age >55 years showed significantly lower evoked amplitudes.
What is the implication, and what should change now?
• Surgeons should think various clinical factors when interpreting electromyographic data in thyroid intraoperative neuromonitoring using skin electrodes.
Introduction
Intraoperative neuromonitoring (IONM) has been reported to help identify the nerve early, safely dissect the nerve, and predict postoperative vocal cord movements in thyroid surgery (1). Currently, the most popular electromyography (EMG) signal recording method for thyroid IONM is to use an endotracheal tube with surface electrodes (EMG tube), which has been the best established to date and is easy to use if the equipment is ready (1). However, the EMG tube has the disadvantage of false-positive loss of signal (LOS) due to poor contact between the EMG tube and vocal folds (2). IONM using needle or skin electrodes has been proposed as an alternative method (3-8). Among them, the skin electrode is the most noninvasive and free of false-positive LOS due to tube malposition and has an economic advantage compared to the EMG tube (6-8). However, skin electrodes have the disadvantage that the evoked amplitude value is relatively low and an appropriate standard value has not yet been established.
In this study, we analyzed normative thyroid IONM data recorded via skin electrodes and factors, such as age, sex, side and extent of surgery, and body mass index (BMI), which could affect the evoked amplitude of thyroid IONM. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-428/rc).
Methods
Patients
This retrospective study reviewed the medical records of 167 consecutive patients with 242 nerves at risk (NAR) who underwent conventional thyroid lobectomy or total thyroidectomy (TT) with IONM using skin electrodes between May, 2020 and June, 2021. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Pusan National University Hospital approved this study (No. 1910-042-084) and waived the requirement for informed consent. All enrolled patients showed normal glottic function on preoperative laryngoscopic examination. Clinicopathological characteristics, such as age, sex, BMI, surgical extent, tumor size, and tumor pathology, were analyzed (Table 1).
Table 1
| Clinicopathologic characteristics | Value |
|---|---|
| Number of patients | 167 |
| NAR, n | 242 |
| Type of surgery, n (hemithyroidectomy/total thyroidectomy) |
92/75 |
| Age (mean ± SD, years) | 51.92±12.53 |
| Sex (M:F), n | 22:145 |
| Body mass index (mean ± SD, kg/m2) | 24.38±3.09 |
| Tumor size (mean ± SD, cm) | 0.86±0.64 |
| Pathology, n | |
| Papillary thyroid carcinoma | 137 |
| Follicular neoplasm | 16 |
| Benign thyroid disease | 14 |
NAR, nerves at risk; SD, standard deviation; M, male; F, female.
General anesthesia and monitor setup
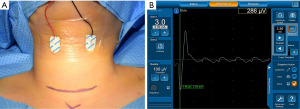
After standard general anesthesia, anesthesia was maintained with sevoflurane, and neuromuscular blockade was not administered during surgery. All patients were placed in the Rose position and a pair of skin electrodes (DSE3125, Medtronic Xomed Inc., Jacksonville, FL, USA) was attached to the lateral border of the thyroid cartilage lamina (Figure 1A). The NIM-Neuro 3.0 system (Medtronic Xomed Inc.) was used for IONM analysis. The duration of stimulation was set at 100 µS, with a frequency of 4 Hz. Recurrent laryngeal nerve (RLN) and vagus nerve (VN) stimulation was performed after extraction of the thyroid specimen, and evoked EMG data, including mean amplitude and latency, were recorded (Figure 1B). Furthermore, the recorded EMG values were compared according to clinical factors, such as age, sex, side and extent of surgery, and BMI.
Statistical analysis
The collected EMG data were expressed as mean ± standard error and compared for each variable using a paired t-test and one-way analysis of variance. Multiple regression analysis was performed to assess the effects of age and BMI on EMG data. Statistical significance was set at P<0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences version 27 for Windows (IBM Corp., Armonk, NY, USA).
Results
A total of 167 patients with 242 NARs were included in the study. The ratio of hemithyroidectomy (HT) to TT was 92:75. The mean age of the patients was 51.92±12.53 years (range, 24−78 years). Among the 167 patients, 137 had papillary thyroid carcinoma, 16 had follicular neoplasm, and 14 had benign thyroid disease. The mean tumor size was 0.86±0.64 cm. The mean BMI was 24.38±3.09 kg/m2.
The mean amplitude of both RLN and VN recorded via skin electrodes was 264.37±101.49 and 243.71±77.82 µV, respectively. The mean latency of the right and left VN was 5.37±0.08 and 7.57±0.10 mS, respectively. There were no cases of LOS in the current study.
Comparison of EMG data according to surgical side
Of the 242 NARs, 132 were left and 110 were right. The mean amplitude of the left and right VN was 241.95±79.45 and 249.97±76.83 µV, respectively. The mean latency of the left and right VN was 7.57±0.10 and 5.37±0.08 mS, respectively. The mean amplitude of the left and right RLN was 270.45±104.87 and 262.31±98.96 µV, respectively. The mean latency of the left and right RLN was 3.49±0.08 and 3.22±0.03 mS, respectively. There was no statistical difference in the amplitude value according to the surgical side but a statistical difference in the VN latency according to the surgical side (P<0.01) (Table 2).
Table 2
| Parameters | Nerve | Subtypes | NAR (n) | Amplitude (mean ± SD, μV) |
P value | Latency (mean ± SD, mS) |
|---|---|---|---|---|---|---|
| Side | VN | Left | 132 | 241.95±79.45 | 0.84 | 7.57±0.10 |
| Right | 110 | 249.97±76.83 | 5.37±0.08** | |||
| RLN | Left | 132 | 270.45±104.87 | 0.76 | 3.49±0.08 | |
| Right | 110 | 262.31±98.96 | 3.22±0.03 | |||
| Sex | VN | Male | 38 | 200.50±57.12*** | <0.001 | 6.44±0.10 |
| Female | 204 | 254.35±79.18 | 6.39±0.13 | |||
| RLN | Male | 38 | 196.05±77.68*** | <0.001 | 3.73±0.13 | |
| Female | 204 | 280.44±101.49 | 3.58±0.09 | |||
| Surgical extent | VN | HT | 92 | 261.89±88.49 | 0.01 | 6.68±1.54 |
| TT | 150 | 236.15±69.72* | 6.62±1.44 | |||
| RLN | HT | 92 | 285.57±108.82 | 0.02 | 3.42±0.65 | |
| TT | 150 | 255.48±96.53* | 3.25±0.50 | |||
| Age (years) | VN | <55 | 113 | 266.44±89.69 | <0.001 | 6.25±1.49 |
| ≥55 | 129 | 226.44±60.39*** | 6.35±1.55 | |||
| RLN | <55 | 113 | 291.39±110.67 | <0.001 | 3.32±0.72 | |
| ≥55 | 129 | 244.44±88.37*** | 3.35±0.48 |
Statistical difference: *, P<0.05; **, P<0.01; ***, P<0.001. NAR, nerves at risk; SD, standard deviation; VN, vagus nerve; RLN, recurrent laryngeal nerve; HT, hemithyroidectomy; TT, total thyroidectomy.
Comparison of EMG data according to sex
Of the 242 NARs, 38 were from men, and 204 were from women. The mean amplitude values of VN according to sex were as follows: men, 200.50±57.12 µV and women, 254.35±79.18 µV (P<0.05). The mean latency values of the VN according to sex were as follows: men, 6.44±0.10 mS and women, 6.39±0.13 mS. The mean amplitude of RLN for men and women was 196.05±77.68 and 280.44±101.49 µV, respectively. The mean latency of RLN for men and women was 3.73±0.13 and 3.58±0.09 mS, respectively. There were no significant differences in latency according to sex in RLN and VN. Otherwise, there was a statistically significant difference in the amplitude value according to the surgical side in the RLN and VN (P<0.001) (Table 2).
Comparison of EMG data according to surgical extent
Of the 242 NARs, 92 were in the HT group and 150 in the TT group. The mean amplitude values of both VN and RLN according to the surgical extent were the following: the VN HT group, 261.89±88.49 µV; VN TT group, 236.15±69.72 µV; RLN HT group, 285.57±108.82 µV; and RLN TT group, 255.48±96.53 µV. The mean latency values of both VN and RLN according to the surgical extent were as follows: the VN HT group, 6.68±1.54 mS; VN TT group, 6.62±1.44 mS; RLN HT group, 3.42±0.65 mS; and RLN TT group, 3.25±0.50 mS. There were no significant differences in latency according to BMI in both RLN and VN. However, the mean amplitude value of the TT group was significantly lower than that of the HT group for the VN and RLN (P<0.05) (Table 2).
Comparison of EMG data according to BMI
We classified the patient group according to the Asia-Pacific BMI classification (normal, <18.5 kg/m2; overweight, 18.5–22.9 kg/m2; and obesity, ≥23 kg/m2). Of the 242 NARs, 93 were in the normal group, 59 in the overweight group, and 90 in the obesity group. The mean amplitude values of both VN and RLN according to BMI were as the following: the VN normal group, 261.58±68.57 µV; VN overweight group, 240.85±90.62 µV; VN obesity group, 226.89±75.44 µV; RLN normal group, 288.86±95.36 µV; RLN overweight group, 272.56±116.87 µV; and RLN obesity group, 233.97±90.50 µV. The three groups showed significant differences in mean amplitude values. The mean amplitude values of VN (P<0.05) and RLN (P<0.01) in the obese group were significantly lower than those of the normal group (Table 3). In multiple regression analyses, as BMI increased, the RLN amplitude tended to decrease significantly (P<0.01) (Table 4). Similarly, the VN amplitude tended to decrease with increasing BMI, but no statistical significance was observed (Table 5).
Table 3
| Nerve | BMI group† | NAR | Amplitude (mean ± SD, μV) |
F | P value | Scheffe |
|---|---|---|---|---|---|---|
| VN | Normal (a) | 93 | 261.58±68.57 | 4.161 | 0.017* | a<c |
| Overweight (b) | 59 | 240.85±90.62 | ||||
| Obesity (c) | 90 | 226.89±75.44 | ||||
| RLN | Normal (a) | 93 | 288.86±95.36 | 6.403 | 0.002** | a<c |
| Overweight (b) | 59 | 272.56±116.87 | ||||
| Obesity (c) | 90 | 233.97±90.50 |
†, categories are based on Asia-Pacific BMI classification: normal, <18.5 kg/m2; overweight, 18.5−22.9 kg/m2; obesity, ≥23 kg/m2. Statistical difference: *, P<0.05; **, P<0.01. BMI, body mass index; NAR, nerves at risk; SD, standard deviation; VN, vagus nerve; RLN, recurrent laryngeal nerve; F, F-static.
Table 4
| Variables | Unstandardized coefficients | Standardized coefficients (β) | T (P value) | Collinearity statistics | ||
|---|---|---|---|---|---|---|
| B | SE | Tolerance | VIF | |||
| (Constant) | 479.475 | 63.171 | 7.590 (<0.001)*** | |||
| BMI | −6.180 | 2.227 | −0.188 | −2.775 (0.006)** | 0.997 | 1.003 |
| Age | −1.236 | 0.551 | −0.152 | −2.246 (0.026)* | 0.997 | 1.003 |
| Adjusted R square | 0.046 | |||||
| Durbin-Watson | 1.986 | |||||
Statistical difference: *, P<0.05; **, P<0.01; ***, P<0.001. BMI, body mass index; B, beta; SE, standard error; T, t-static; VIF, variance inflation factor.
Table 5
| Variables | Unstandardized coefficients | Standardized coefficients (β) | T (P value) | Collinearity statistics | ||
|---|---|---|---|---|---|---|
| B | SE | Tolerance | VIF | |||
| (Constant) | 353.674 | 49.189 | 7.190 (<0.001)*** | |||
| BMI | −0.724 | 0.429 | −0.116 | −1.689 (0.093) | 0.997 | 1.003 |
| Age | −2.962 | 1.734 | −0.118 | −1.708 (0.089) | 0.997 | 1.003 |
| Adjusted R square | 0.016 | |||||
| Durbin-Watson | 1.847 | |||||
Statistical difference: ***, P<0.001. BMI, body mass index; B, beta; SE, standard error; T, t-static; VIF, variance inflation factor.
Comparison of EMG data according to age
According to the American Joint Committee on Cancer staging system, patients were classified according to the age of 55 years. Of the 242 NARs, 113 were aged <55 years and 129 were aged ≥55 years. The mean amplitude values of both VN and RLN according to the age group were as follows: the VN aged <55 group, 266.44±89.69 µV; VN aged ≥55 group, 226.44±60.39 µV; RLN aged <55 group, 291.39±110.67 µV; and RLN aged ≥55 group, 244.44±88.37 µV. The patients aged ≥55 years showed significantly lower amplitude values than those aged <55 years (P<0.001) (Table 2). In multiple regression analyses, as age increased, the RLN amplitude tended to decrease significantly (P<0.05) (Table 4). The VN amplitude decreased with age, but the difference was not statistically significant (Table 5).
Discussion
Wu et al. reported for the first time the results of thyroid IONM using adhesive skin electrodes (8). Using porcine models, although the evoked amplitude value of the adhesive skin electrode was lower than that of the EMG tube, IONM could be successfully performed without false positives, and the stress injury of the RLN could be evaluated identically to that of the EMG tube. Furthermore, unlike the EMG tube, which showed a false LOS when there was a trachea displacement, the adhesive skin electrode showed a stable signal (8). Lee et al. successfully performed thyroid IONM in human patients using adhesive skin electrodes. In their study, some NARs showed amplitudes of ≤100 µV, but all showed biphasic waveforms (6). Liddy et al. performed thyroid IONM using an anterior laryngeal electrode attached to the thyroid cartilage; their study showed that the amplitude was relatively lower (up to 83%) than the EMG tube, but there were no differences in latency (9). Previously, we compared the EMG data recorded using the EMG tube and the skin electrode simultaneously in the same patient (7). The amplitude of the skin electrode was approximately one-third of the EMG tube.
Previous studies have shown that skin electrodes have several advantages: (I) they are noninvasive; (II) they are free from false-positive LOS due to tube malposition; and (III) they are economical (6-8). Unfortunately, the adhesive skin electrode does not have as many previous research results as the EMG tube, so the normative value ranges and event threshold have not yet been established.
In this study, the normative values of thyroid IONM using adhesive skin electrodes and factors that could affect the amplitude values were analyzed. IONM data were obtained for all NARs without a false LOS during the study period. The mean amplitude of both RLN and VN recorded via skin electrodes was 264.37±101.49 and 243.71±77.82 µV, respectively. These values correspond to approximately 32–35% of the amplitude of the EMG tube (7,10). Furthermore, the mean latency of the right VN was 5.37±0.80 mS and the left VN was 7.57±1.02 mS. These values correspond to approximately 137% of the latency of the EMG tube (7,10). The normative value of the current study was only one-third of the EMG tube amplitude value compared to previous reports (7,10,11). This assumes that the distance between the vocal cord muscle where the EMG signal is generated and the skin electrode that records the EMG signal is relatively far compared to the EMG tube; thus, signal attenuation occurs due to barriers, such as cartilage, muscle, and fat, that could increase resistance.
Factors affecting the values of the EMG data, such as surgical side, surgical extent, sex, BMI, and correlations were analyzed. The amplitude tended to decrease significantly with greater surgical extent, male sex, obesity, and age ≥55 years. These clinical factors may have confounding factors each other. However, the reason for this tendency is assumed to be that the thickness of the neck tends to increase in men and obese people, and as a result, the distance between the adhesive skin electrode and the vocal cord muscles increases, resulting in EMG signal attenuation. Furthermore, the amplitude was significantly lower in the TT group than in the HT group, which is believed to be related to the flap elevation range. The resistance between the adhesive skin electrode and vocal cord muscles increased as the flap elevation range increased, causing signal attenuation.
This study has the limitations such as retrospective, non-comparative design, insufficient number of cases. And although skin electrodes are useful, the standard IONM method so far is the EMG tube.
Conclusions
In conclusion, the mean amplitude of both RLN and VN recorded via skin electrodes was 264.37±101.49 and 243.71±77.82 µV, respectively, which corresponded to approximately one-third of the EMG tube. The factors influencing the amplitude values were sex, surgical extent, BMI, and age. Male sex, larger surgical extent, obesity, and age ≥55 years showed significantly lower amplitude values.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-428/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-428/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-428/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-428/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Pusan National University Hospital approved this study (No. 1910-042-084) and waived the requirement for informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Randolph GW, Dralle H, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 2008;32:1358-66.
- Lee HS, Seo SG, Kim DY, et al. Intraoperative Neuromonitoring Using a Single Transcartilage Needle Electrode During Thyroidectomy. Laryngoscope 2021;131:448-52. [Crossref] [PubMed]
- Wu CW, Chiang FY, Randolph GW, et al. Feasibility of Intraoperative Neuromonitoring During Thyroid Surgery Using Transcartilage Surface Recording Electrodes. Thyroid 2018;28:1508-16. [Crossref] [PubMed]
- Jung SM, Tae K, Song CM, et al. Efficacy of Transcartilaginous Electrodes for Intraoperative Neural Monitoring During Thyroid Surgery. Clin Exp Otorhinolaryngol 2020;13:422-8. [Crossref] [PubMed]
- Lee HS, Oh J, Kim SW, et al. Intraoperative Neuromonitoring of Recurrent Laryngeal Nerve During Thyroidectomy with Adhesive Skin Electrodes. World J Surg 2020;44:148-54. [Crossref] [PubMed]
- Shin SC, Sung ES, Kwon HK, et al. Investigation of attachment location of adhesive skin electrodes for intraoperative neuromonitoring in thyroid surgery: Preclinical and clinical studies. Surgery 2022;171:377-83. [Crossref] [PubMed]
- Wu CW, Chiang FY, Randolph GW, et al. Transcutaneous Recording During Intraoperative Neuromonitoring in Thyroid Surgery. Thyroid 2018;28:1500-7. [Crossref] [PubMed]
- Liddy W, Lawson BR, Barber SR, et al. Anterior laryngeal electrodes for recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: New expanded options for neural monitoring. Laryngoscope 2018;128:2910-5. [Crossref] [PubMed]
- Lorenz K, Sekulla C, Schelle J, et al. What are normal quantitative parameters of intraoperative neuromonitoring (IONM) in thyroid surgery? Langenbecks Arch Surg 2010;395:901-9. [Crossref] [PubMed]
- Chiang FY, Lu IC, Chang PY, et al. Comparison of EMG signals recorded by surface electrodes on endotracheal tube and thyroid cartilage during monitored thyroidectomy. Kaohsiung J Med Sci 2017;33:503-9. [Crossref] [PubMed]


