Mucoepidermoid carcinoma—a rare salivary gland-type tumor of the breast: are we dealing with primary or secondary?—a case report and literature review
Highlight box
Key findings
• We report a rare case of estrogen-receptor positive mucoepidermoid carcinoma (MEC) of the breast in a patient with salivary gland tumor history.
What is known and what is new?
• MEC is a tumor most commonly occurring in the salivary glands. MEC of the breast is a rare, predominantly triple-negative breast cancer with a relatively favorable prognosis but little consensus on ideal treatment modality.
• No other cases of MEC of the breast in a patient with salivary gland tumor history have been reported. The current report highlights the importance of accurate diagnosis and appropriate surgical and adjuvant treatments.
What is the implication, and what should change now?
• The early and precise diagnosis of MEC of the breast, including evaluation of possible associated tumors, is crucial to formulating effective treatment strategies and ensuring positive survival rates.
Introduction
Mucoepidermoid carcinoma (MEC) is an invasive tumor of the breast that histologically resembles its salivary gland counterpart (1). Since its initial documentation in 1979, approximately 47 cases have been reported in the English literature to date (2,3). The estimated incidence of MEC accounts for 0.2–0.3% of all breast tumors; however, some authors believe that the true incidence may be higher due to the potential misclassification of cases as carcinomas with squamous differentiation (4,5). Furthermore, despite being most frequently detected in the salivary gland, MEC has been reported to occur in a variety of organs, including the lungs, bronchus, esophagus, and thyroid (6). Regardless of location, the morphology of this tumor is characterized by a mixture of mucinous, squamous, and intermediate neoplastic cells arranged in solid and cystic patterns (1,6). Based on the tumor’s histomorphology, MEC can be categorized into low, intermediate, or high grades (7). Regardless of the grade, the cell composition is similar. Low-grade MEC tend to be more cystic, while high-grade MEC is more solid with a high nuclear grade, necrosis, and brisk mitotic figures (8,9). To identify these aforementioned cell types observed in MEC, several immunohistochemical stains are utilized. CK14 stains basaloid cells, p63 stains epidermoid cells, and CK7 delineates mucous cells (9). Moreover, using GATA3 and mammaglobin expression, these stains help distinguish MEC of the breast from MEC of the salivary, where the former will be expressed in breast MEC and negative in the latter (3).
While most documented cases in the literature emphasize MEC as a prevalent form of triple-negative breast cancer, these cases generally exhibit low invasiveness and a favorable prognosis (10). In fact, tumor grade has been identified as the most important predictor of long-term prognosis in MEC patients. Currently, there is no consensus or standard therapeutic guideline for the treatment of MEC. Prior studies have suggested that high-grade MEC is typically managed through mastectomy and axillary lymph node dissection, while breast conservation and sentinel node biopsy may be options for tumors of low and intermediate grade. However, a significant portion of these studies did not account for the hormone receptor status of patients, and those that did reported a prevalence of triple-negative breast cancer phenotypes. Consequently, there exists a scarcity of literature that examines the role of hormone receptor status on treatment outcomes (3,11). Thus, our case report aimed to underscore the diagnostic process, surgical and adjunctive treatments for our patient with estrogen receptor (ER)-positive, progesterone receptor (PR)-negative, human epidermal growth factor receptor 2 (HER2)-negative MEC, while also conducting a literature review to contribute to the limited existing data. We present this case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-372/rc).
Case presentation
A 67-year-old postmenopausal African American woman presented with a lobulated 3 cm left breast mass on screening mammogram. She denied any nipple inversion, nipple discharge or skin changes. Diagnostic mammogram and ultrasound demonstrated a 3.1 cm mass at 2:00 o’clock 9 cm from the nipple and an abnormal left axillary node with a thickened cortex. Subsequently, an ultrasound-guided core biopsy revealed carcinoma with squamous differentiation, ER-positive (51–60%), PR-negative, and HER2-negative by fluorescence in situ hybridization (FISH). Biopsy of the left axillary lymph node was benign. A follow-up magnetic resonance imaging (MRI) confirmed the tumor at 2:00 o’clock about 6.7 cm from the nipple measuring 2.1 cm × 2.9 cm × 2.8 cm with biopsy clip and no additional sites of disease. She had a past medical history significant for a 10-year history of a slowly enlarging right parotid mass, for which she underwent right deep lobe parotidectomy with facial nerve dissection and preservation at age 51. Pathology report of the excised mass revealed a 2.3-cm pleomorphic adenoma, with no features suggestive of malignant transformation. One intraparotid and two right neck lymph nodes were also negative. Her family history included breast cancer in her daughter, who was diagnosed in her late thirties and was found to have a pathogenic BReast CAncer gene 1 (BRCA1) variant. However, the patient’s genetic testing results were negative.
The patient was presented at our institutional multidisciplinary tumor board with recommendations to undergo left breast lumpectomy and left sentinel lymph node biopsy.
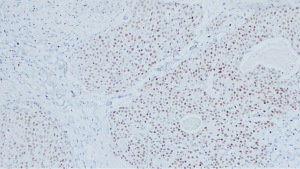
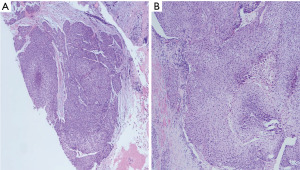
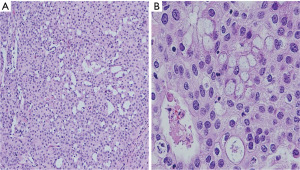
A left breast lumpectomy with margin assessment and sentinel lymph node biopsy was performed. The patient had four sentinel lymph nodes sent for final pathology. The surgery was successful, and the patient tolerated the procedure well. There were no intraoperative or postoperative complications. After an uneventful recovery, she was discharged the same day. Final pathology revealed the presence of 33 mm × 30 mm × 25 mm, grade-2 (as per salivary grading system) stage IIA MEC without angiolymphatic or perineural invasion that had been fully removed with clear margins. The tumor tested positive for ER but negative for PR and HER-2. Histologically, the tumor comprised of irregular nests of intermediate tumor cells with squamous differentiation (Figure 1) and mucous cells (Figures 2,3). It stained positive for GATA3 (diffuse, weak) (Figure 4) and ER. Additionally, as part of her adjuvant treatment, the patient was referred to medical oncology and radiation oncology for adjuvant treatment recommendations. She underwent 15 fractions of external beam radiation (48 Gy) and was stated on adjuvant aromatase inhibitor.
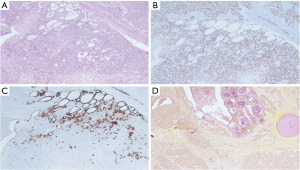
On follow-up, the patient was noted to have an enlarging neck mass for which she underwent a positron emission tomography (PET) scan and subsequent fine needle aspiration. While the PET scan showed increased focal uptake in the left inferior thyroid lobe, cytological test demonstrated numerous lymphoid cells and scattered oncocytic cells, negative for malignancy.
The total length of post-operative follow-up was 7 months. The patient’s is alive with no evidence of disease. No recurrence or metastasis were reported during the follow-up period.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Primary MEC of the breast is a rare, atypical tumor, accounting for only 0.2–0.3% of all primary breast tumors (4). The first cases were reported in 1979 by Patchefsky et al. (2) and since then 47 cases have been reported (2,7). MEC morphology can be heterogenous. Therefore, it is often confused with other benign and malignant neoplasms (6). All 47 reported cases have occurred in adult women with a wide age range of 29 to 80 years and a mean age of 55.7. None of the previously reported cases reported presence of BRCA gene positivity, but of note, our patient reported a family history of BRCA1 positivity in her daughter, who was diagnosed with breast cancer in her late thirties. Furthermore, our patient had a history of pleomorphic adenoma removal at age 51. The significance of this benign salivary gland tumor history is unclear. Given MEC is a common salivary tumor with varying potential for aggressive behavior, there was suspicion to whether the patient’s MEC tumor of the breast was a primary or secondary tumor (12,13). Since pleomorphic adenomas harbor a small risk of malignant transformation, it was determined the patient’s MEC of the breast was likely a primary tumor.
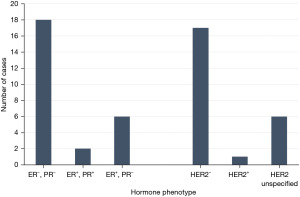
Although the majority of MEC cases have been documented in the United States (11 cases) (2,4-6,14), followed by Italy (6 cases) (15,16), China (6 cases) (9,11,17), and Turkey (3 cases) (18-20), none have provided insights into the racial or ethnic backgrounds of their patients. To the best our knowledge, we report the first case of MEC in an African American patient. Multiple studies have demonstrated that breast MEC presents as a triple-negative cancer (7,9). Contrastingly, our patient showed positivity for ER (60%) and absence of PR and HER2. Moreover, in terms of receptor status, 12 studies were triple-negative (4,7,9,14,19,21-25). Six were ER−, PR−, and HER2 unspecified (26-30). In cases not classified as triple negative, three were ER+, PR−, and HER2− including our case (8,11); two were ER+, PR+, HER2−. This has been further illustrated in Figure 5. Recent cases have shown an increasing incidence of non-triple negative breast cancers (6,8,21). The hypothesis that hormone receptor plays a role in prognosis was corroborated by Sherwell-Cabello et al. (21), who found favorable outcomes associated with lower hormone receptor expression. This finding suggests a potential hormone dependency of the disease and raises the possibility of considering endocrine therapy as a viable option.
Histological grade is an important prognostic factor in MEC of the breast (23). This type of tumor is graded using either the salivary gland grading system or the breast grading system, both yielding comparable outcomes. Among these grading frameworks, the Elston Ellis scoring system takes prominence. This system effectively categorizes tumors into low, intermediate, and high grades, factoring in components like cystic proportion, nerve invasion, necrosis, as well as the count of mitoses per 10 high-power fields (9,31). Patients diagnosed with high-grade MEC face a less favorable prognosis, often experiencing the development of distant metastases (9). Of the reported cases with low and intermediate grade, no deaths were reported which supported the hypothesis that low and intermediate grade MEC had a favorable clinical outcome (11,32). Low-grade MEC is non-aggressive, whereas high-grade MEC is aggressive, frequently leading to metastasis in axillary lymph nodes and distant organs (9,14,15).
To better understand the pathogenesis of MEC in salivary versus extra-salivary origins, researchers have compared the molecular profile of primary breast MECs with salivary and extra-salivary MECs (33). Primary MEC in the salivary gland and lung have been found to be associated with MAML2 fusion (34,35), however, the pathogenesis of primary thyroid MEC seems to be MAML2-independent (36). Among the reported cases of breast MEC that have been investigated for presence of MAML2 rearrangements, three have presented with the feature, suggesting a need for the classification of more tumors (1,14).
In the present literature, the standard surgical approach for MEC of the breast has not been well established because of its low incidence. Our patient underwent a lumpectomy for an intermediate-grade tumor measuring 2.9 cm. In cases documented prior to 2000, most patients underwent mastectomy or modified radical mastectomy (14 cases), regardless of tumor size or grade, while only three cases mentioned breast-conserving procedures such as quadrantectomy, lumpectomy, or wide local excision. Post-2000, the majority of reported cases involved mastectomies or modified radical mastectomies (18 cases), with six cases reporting lumpectomies or local excisions, and three cases involving quadrantectomies (Table 1). This trend indicates a growing number of surgeons opting for breast-conserving surgery for removing breast MEC tumors, although mastectomies continue to be the preferred treatment option.
Table 1
| Case No. | Author (ref.) | Year | Surgical approach | Adjuvant therapy | Follow-up (mo.) | Status |
|---|---|---|---|---|---|---|
| Present case | Zhang et al. | 2023 | Lumpectomy + SLD | Radiation, hormonal | 6 | Alive |
| 1 | Gupta et al. (7) | 2023 | MRM | None | NA | Alive |
| 2 | Bak et al. (8) | 2022 | Lumpectomy + SLD | Chemotherapy, radiation, hormonal | 37 | Alive |
| 3 | Chen et al. (17) | 2022 | Excision | NA | 6 | Alive |
| 4 | Bui et al. (6) | 2022 | Lumpectomy + SLD | Radiation, hormonal | NA | Alive |
| 5 | Ye et al. (9) | 2020 | MRM | Chemotherapy | 12 | Alive |
| 6 | Yan et al. (14) | 2020 | Lumpectomy | NA | 60 | Alive |
| 7 | Burghel et al. (10) | 2018 | NA | None | NA | NA |
| 8 | Sherwell-Cabello et al. (21) | 2017 | MRM | None | 3 | Alive |
| 9 | Cheng et al. (11) | 2017 | MRM | NA | 156 | Alive |
| 10 | MRM | NA | 41 | Alive | ||
| 11 | Mastectomy + SLD | NA | 9 | Alive | ||
| 12 | Mastectomy + SLD | NA | 4 | Alive | ||
| 13 | Arun Kumar et al. (22) | 2016 | MRM | Chemotherapy, radiation, hormonal | 24 | Alive |
| 14 | Fujino et al. (23) | 2016 | Mastectomy + SLD | NA | NA | NA |
| 15 | Palermo et al. (24) | 2013 | NA | NA | NA | NA |
| 16 | Turk et al. (18) | 2013 | MRM | NA | 5 | Alive |
| 17 | Basbug et al. (19) | 2011 | MRM | Chemotherapy, radiation | 12 | Alive |
| 18 | Camelo-Piragua et al. (4) | 2009 | MRM | Chemotherapy | 8 | Alive |
| 19 | Hornychová et al. (25) | 2007 | SM + LND | Chemotherapy, radiation | 18 | Alive |
| 20 | MRM | Chemotherapy, radiation | 60 | Alive | ||
| 21 | Horii et al. (32) | 2006 | Mastectomy + LND | Hormonal | 36 | Alive |
| 22 | Gómez-Aracil et al. (37) | 2006 | MRM + LND | NA | 54 | Alive |
| 23 | Di Tommaso et al. (15) | 2004 | Excision | NA | 5 | Alive |
| 24 | Excision | NA | 90 | Alive | ||
| 25 | Quadrantectomy + LND | NA | 13 | Alive | ||
| 26 | Quadrantectomy + LND | NA | 3 | Alive | ||
| 27 | Quadrantectomy + LND | NA | 18 | Alive | ||
| 28 | Terzi et al. (20) | 2004 | MRM | NA | NA | NA |
| 29 | Tjalma et al. (30) | 2002 | RM | None | 156 | Alive |
| 30 | Berry et al. (38) | 1998 | Mastectomy + LND | NA | NA | NA |
| 31 | Markopoulos et al. (29) | 1998 | Wide local excision + LND | Chemotherapy, radiation, hormonal | 60 | Alive |
| 32 | Chang et al. (26) | 1998 | MRM | Chemotherapy | 48 | Alive |
| 33 | Lüchtrath and Moll (39) | 1989 | RM | NA | 30 | DOD |
| 34 | Pettinato et al. (16) | 1989 | MRM | NA | 10 | DOD |
| 35 | Hanna and Kahn (27) | 1985 | MRM | NA | 8 | Alive |
| 36 | MRM | NA | 14 | Alive | ||
| 37 | Hastrup and Sehested (28) | 1985 | RM | NA | 25 | DOD |
| 38 | Leong and Williams (40) | 1985 | SM | NA | 7 | DOD |
| 39 | Ratanarapee et al. (41) | 1983 | NA | NA | 14 | DOD |
| 40 | Fisher et al. (5) | 1983 | Lumpectomy | NA | 60 | Alive |
| 41 | MRM | NA | 48 | Alive | ||
| 42 | MRM | NA | 120 | Alive | ||
| 43 | RM | NA | 108 | Alive | ||
| 44 | SM | NA | 48 | Alive | ||
| 45 | Kovi et al. (42) | 1981 | MRM | NA | NA | NA |
| 46 | Patchefsky et al. (2) | 1979 | RM | None | 94 | DOR |
| 47 | Quadrantectomy | None | 10 | Alive |
SLD, sentinel lymph node; MRM, modified radical mastectomy; NA, not applicable; SM, simple mastectomy; LND, lymph node dissection; RM, radical mastectomy; DOD, died of disease; DOR, died of other reasons.
Lastly, there are no established guidelines for adjuvant therapy for treatment of MEC. Prior studies have documented a range of approaches, including chemotherapy, radiation, hormonal therapy, different combinations of these methods, or no additional treatment. Except for one patient who did not receive any adjuvant therapy and died due to unrelated causes, all patients who received adjuvant therapy survived until the end of the follow-up period. As noted earlier, the prognosis of breast MEC remains dependent on the pathological grade of the tumor, and the role of adjuvant therapy remains unclear. Therefore, future studies with a larger sample size are needed to explore the role of adjuvant therapy in MEC. Furthermore, additional studies are also required to better understand the significance of hormone receptor status in the context of MEC.
A limitation of this case study is its short length of follow-up (6 months). Additionally, to our knowledge, this is the first and only case of MEC of the breast reported at our institution. Therefore, we are unable to comment on whether breast-conserving surgery and adjuvant therapy is the best approach to treatment. Nevertheless, MEC of the breast has relatively good prognosis, as none of the intermediate grade lesions, similar to the present study, led to distant metastasis or death (4,8,23).
Conclusions
MEC of the breast is a rare tumor with a relatively favorable overall prognosis. The early and precise diagnosis of this condition plays a pivotal role in formulating effective treatment strategies and ensuring positive survival rates. Nonetheless, future studies are recommended to further explore the role of surgical approaches and adjuvant therapy to improve treatment outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-372/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-372/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-372/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bean GR, Krings G, Otis CN, et al. CRTC1-MAML2 fusion in mucoepidermoid carcinoma of the breast. Histopathology 2019;74:463-73. [Crossref] [PubMed]
- Patchefsky AS, Frauenhoffer CM, Krall RA, et al. Low-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med 1979;103:196-8.
- Joneja U, Palazzo J. The Spectrum of Mucinous Lesions of the Breast. Arch Pathol Lab Med 2023;147:19-29. [Crossref] [PubMed]
- Camelo-Piragua SI, Habib C, Kanumuri P, et al. Mucoepidermoid carcinoma of the breast shares cytogenetic abnormality with mucoepidermoid carcinoma of the salivary gland: a case report with molecular analysis and review of the literature. Hum Pathol 2009;40:887-92. [Crossref] [PubMed]
- Fisher ER, Palekar AS, Gregorio RM, et al. Mucoepidermoid and squamous cell carcinomas of breast with reference to squamous metaplasia and giant cell tumors. Am J Surg Pathol 1983;7:15-27. [Crossref] [PubMed]
- Bui CM, Bose S. Primary mucoepidermoid carcinoma of the breast arising in adenomyoepithelioma. BMJ Case Rep 2022;15:e247281. [Crossref] [PubMed]
- Gupta RK, Agrawal M, Simon A, et al. Mucoepidermoid Carcinoma of Breast Presenting as Abscess-like Pus Discharging Mass; A Rare Tumour With Unusual Presentation. Arch Breast Cancer 2023;10:200-4.
- Bak S, Choi HY, Lee JH, et al. Mucoepidermoid carcinoma of the breast: A case report and literature review focused on radiological findings. Medicine (Baltimore) 2022;101:e29745. [Crossref] [PubMed]
- Ye RP, Liao YH, Xia T, et al. Breast mucoepidermoid carcinoma: a case report and review of literature. Int J Clin Exp Pathol 2020;13:3192-9.
- Burghel GJ, Abu-Dayyeh I, Babouq N, et al. Mutational screen of a panel of tumor genes in a case report of mucoepidermoid carcinoma of the breast from Jordan. Breast J 2018;24:1102-4. [Crossref] [PubMed]
- Cheng M, Geng C, Tang T, et al. Mucoepidermoid carcinoma of the breast: Four case reports and review of the literature. Medicine (Baltimore) 2017;96:e9385. [Crossref] [PubMed]
- Bai S, Clubwala R, Adler E, et al. Salivary mucoepidermoid carcinoma: a multi-institutional review of 76 patients. Head Neck Pathol 2013;7:105-12. [Crossref] [PubMed]
- Valstar MH, Mast H, Ten Hove I, et al. Malignant transformation of salivary gland pleomorphic adenoma: proof of principle. J Pathol Clin Res 2021;7:432-7.
- Yan M, Gilmore H, Harbhajanka A. Mucoepidermoid Carcinoma of the Breast With MAML2 Rearrangement: A Case Report and Literature Review. Int J Surg Pathol 2020;28:787-92. [Crossref] [PubMed]
- Di Tommaso L, Foschini MP, Ragazzini T, et al. Mucoepidermoid carcinoma of the breast. Virchows Arch 2004;444:13-9. [Crossref] [PubMed]
- Pettinato G, Insabato L, De Chiara A, et al. High-grade mucoepidermoid carcinoma of the breast. Fine needle aspiration cytology and clinicopathologic study of a case. Acta Cytol 1989;33:195-200.
- Chen G, Liu W, Liao X, et al. Imaging findings of the primary mucoepidermoid carcinoma of the breast. Clin Case Rep 2022;10:e05449. [Crossref] [PubMed]
- Turk E, Karagulle E, Erinanc OH, et al. Mucoepidermoid carcinoma of the breast. Breast J 2013;19:206-8. [Crossref] [PubMed]
- Basbug M, Akbulut S, Arikanoglu Z, et al. Mucoepidermoid carcinoma in a breast affected by burn scars: comprehensive literature review and case report. Breast Care (Basel) 2011;6:293-7. [Crossref] [PubMed]
- Terzi A, Saglam A, Uner A A. 79 year-old woman with a mass in the right breast. Turk J Cancer 2004;34:38-9.
- Sherwell-Cabello S, Maffuz-Aziz A, Ríos-Luna NP, et al. Primary mucoepidermoid carcinoma of the breast. Breast J 2017;23:753-5. [Crossref] [PubMed]
- Arun Kumar SL, Nagaraja AL, Srinivasaiah M, et al. Mucoepidermoid carcinoma of the breast: a rare case. J Evolution Med Dent Sci 2016;5:739-40.
- Fujino M, Mori D, Akashi M, et al. Mucoepidermoid Carcinoma of the Breast Found during Treatment of Lymphoma. Case Rep Oncol 2016;9:806-14. [Crossref] [PubMed]
- Palermo MH, Pinto MB, Zanetti JS, et al. Primary mucoepidermoid carcinoma of the breast: a case report with immunohistochemical analysis and comparison with salivary gland mucoepidermoid carcinomas. Pol J Pathol 2013;64:210-5. [Crossref] [PubMed]
- Hornychová H, Ryska A, Betlach J, et al. Mucoepidermoid carcinoma of the breast. Neoplasma 2007;54:168-72.
- Chang LC, Lee N, Lee CT, et al. High-grade mucoepidermoid carcinoma of the breast: case report. Changgeng Yi Xue Za Zhi 1998;21:352-7.
- Hanna W, Kahn HJ. Ultrastructural and immunohistochemical characteristics of mucoepidermoid carcinoma of the breast. Hum Pathol 1985;16:941-6. [Crossref] [PubMed]
- Hastrup N, Sehested M. High-grade mucoepidermoid carcinoma of the breast. Histopathology 1985;9:887-92.
- Markopoulos C, Gogas H, Livaditou A, et al. Mucoepidermoid carcinoma of the breast. Eur J Gynaecol Oncol 1998;19:291-3.
- Tjalma WA, Verslegers IO, De Loecker PA, et al. Low and high grade mucoepidermoid carcinomas of the breast. Eur J Gynaecol Oncol 2002;23:423-5.
- Cipriani NA, Lusardi JJ, McElherne J, et al. Mucoepidermoid Carcinoma: A Comparison of Histologic Grading Systems and Relationship to MAML2 Rearrangement and Prognosis. Am J Surg Pathol 2019;43:885-97. [Crossref] [PubMed]
- Horii R, Akiyama F, Ikenaga M, et al. Muco-epidermoid carcinoma of the breast. Pathol Int 2006;56:549-53. [Crossref] [PubMed]
- Venetis K, Sajjadi E, Ivanova M, et al. The molecular landscape of breast mucoepidermoid carcinoma. Cancer Med 2023;12:10725-37. [Crossref] [PubMed]
- Saade RE, Bell D, Garcia J, et al. Role of CRTC1/MAML2 Translocation in the Prognosis and Clinical Outcomes of Mucoepidermoid Carcinoma. JAMA Otolaryngol Head Neck Surg 2016;142:234-40. [Crossref] [PubMed]
- Huo Z, Wu H, Li J, et al. Primary Pulmonary Mucoepidermoid Carcinoma: Histopathological and Moleculargenetic Studies of 26 Cases. PLoS One 2015;10:e0143169. [Crossref] [PubMed]
- Le HT, Nguyen TPX, Hirokawa M, et al. Primary Thyroid Mucoepidermoid Carcinoma (MEC) Is Clinically, Prognostically, and Molecularly Different from Sclerosing MEC with Eosinophilia: A Multicenter and Integrated Study. Endocr Pathol 2023;34:100-11. [Crossref] [PubMed]
- Gómez-Aracil V, Mayayo Artal E, Azua-Romeo J, et al. Fine needle aspiration cytology of high grade mucoepidermoid carcinoma of the breast: a case report. Acta Cytol 2006;50:344-8. [Crossref] [PubMed]
- Berry MG, Caldwell C, Carpenter R. Mucoepidermoid carcinoma of the breast: a case report and review of the literature. Eur J Surg Oncol 1998;24:78-80. [Crossref] [PubMed]
- Lüchtrath H, Moll R. Mucoepidermoid mammary carcinoma. Immunohistochemical and biochemical analyses of intermediate filaments. Virchows Arch A Pathol Anat Histopathol 1989;416:105-13. [Crossref] [PubMed]
- Leong AS, Williams JA. Mucoepidermoid carcinoma of the breast: high grade variant. Pathology 1985;17:516-21. [Crossref] [PubMed]
- Ratanarapee S, Prinyar-Nussorn N, Chantarakul N, et al. High-grade mucoepidermoid carcinoma of the breast. A case report. J Med Assoc Thai 1983;66:642-8.
- Kovi J, Duong HD, Leffall LS Jr. High-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med 1981;105:612-4.