
Clinical effectiveness of microporous polysaccharide hemospheres in mastectomy for patients with breast cancer
Highlight box
Key findings
• Use of microporous polysaccharide hemospheres (MPH) decreased the postoperative drainage volume and drain placement period in mastectomy for patients with breast cancer.
What is known and what is new?
• A previous randomized, controlled study could not demonstrate the effectiveness of MPH in breast surgery, but it was performed in a small cohort (N=50).
• Although our study was retrospective, we analyzed a larger number of patients and showed the effectiveness of MPH in mastectomy for patients with breast cancer.
What is the implication, and what should change now?
• For improving postoperative quality of life, use of MPH could be recommended in mastectomy for patients with breast cancer.
Introduction
Microporous polysaccharide hemospheres (MPH) are hydrophilic polysaccharide particles with a diameter of 30–100 µm that are made from 100% purified potato starch and are currently used as absorbable hemostatic agents. MPH particles extract fluid from the blood, swell to form a gelatinous matrix to concentrate serum proteins, platelets, albumin, thrombin and fibrinogen, and create a scaffold for the formation of a fibrin clot (1,2). Egeli and colleagues reported that MPH could significantly reduce the incidence of seroma after mastectomy and axillary dissection in rats (3). However, the clinical effectiveness of MPH remains controversial. Several randomized, controlled studies could not demonstrate the effectiveness of MPH in patients who underwent breast, thyroid and endoscopic sinus surgery (4-6). In contrast, the effectiveness of MPH in cardiothoracic surgery and total knee arthroplasty has been shown in several retrospective analyses (7,8). In this retrospective study, we evaluated the effectiveness of MPH in breast cancer surgery. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-297/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Kagoshima University Hospital (No. 220041-Epidemiology). Individual consent for this retrospective analysis was waived. Medical records of 352 consecutive patients who underwent total mastectomy for breast cancer at Kagoshima University Hospital were retrospectively reviewed. Between December 2020 and April 2023, we used MPH for all patients who underwent mastectomy with or without axillary dissection (126 patients, MPH group). As a control, we compared the clinical data of 226 patients who underwent mastectomy between January 2015 and November 2020, when we did not use MPH. Patients over 90 years of age and those who underwent breast reconstruction or skin transplantation were excluded. Surgical procedures were total mastectomy, mastectomy with sentinel lymph node biopsy (SLNB) and mastectomy with axillary lymph node dissection (ALND). Ultrasonic or microwave dissectors were used for ALND, depending on the surgeon’s preference, and two drainage tubes were placed for all patients. One gram of MPH (AristaTM AH, C. R. Bard, Inc. Davol, Warwick, RI, USA) was applied to the chest wall and axillary region before wound closure. The drainage tubes were removed when the daily drainage output was below 50 mL per 24 hours, or 14 days after surgery. The collected clinical data were age, height, body weight and body mass index (BMI), and outcomes such as drain placement period duration, drainage output and postoperative complications. Postoperative hemorrhage, seroma formation, wound infection and skin necrosis were recognized as postoperative complications. Postoperative hemorrhage was defined as requiring additional compression after surgery. No patient in our cohort required additional surgery for hemorrhage. Seroma was defined as requiring puncture after drain removal. Wound infection was defined as requiring drainage and/or antibiotic administration, and skin necrosis was defined as wound dehiscence and/or crust formation requiring debridement.
Statistical analysis
Differences between groups were evaluated with the Wilcoxon test for continuous variables and the Pearson Chi-square test for categorical variables. Statistical analysis was performed using JMP Pro, version 16.1.0 for Mac OS (SAS Institute Japan Ltd., Tokyo, Japan). P values <0.05 were considered statistically significant.
Results
Patient characteristics are shown in Table 1. Patients in the MPH group were significantly older (P=0.0021), and received axillary dissection significantly less frequently (P<0.0001) than those in the control group (Table 1). For analysis of the clinical effectiveness of MPH, we analyzed patients with SLNB and ALND separately. For patients with SLNB, there were no significant differences in background characteristics, such as age, height, body weight, BMI, intraoperative blood loss and number of dissected lymph nodes. The drain placement period was significantly shorter and the total drainage volume was significantly smaller in the MPH group compared with the control group (Table 2). Analysis of the variations in the daily amount of drainage found that a significant decrease in drainage volume occurred on postoperative days 2, 3 and 4 in the MPH group compared with the control group (Figure 1). For patients with ALND, age was significantly higher in the MPH group than in the control group. There was no significant difference between groups in patient background characteristics, such as height, body weight, BMI, intraoperative blood loss and number of dissected lymph nodes. The drain placement period was significantly shorter in the MPH group than in the control group, but the total drainage volume did not differ between groups (Table 3). Analysis of the variations in the daily amount of drainage found that a significant decrease in drainage volume occurred on postoperative days 2, 3, 4 and 5 in the MPH group compared with the control group (Figure 2). There was no difference in the frequency of total postoperative complications between the MPH group and the control group, regardless of surgical procedure. When types of complications were analyzed, skin necrosis was significantly less frequent in the MPH group, but the incidence of other complications did not differ between groups (Table 4).
Table 1
| Characteristics | Control (n=226) | MPH (n=126) | P value |
|---|---|---|---|
| Age (years) | 64.4 [29–89] | 68.9 [36–89] | 0.0021 |
| Sex, female/male | 220/6 | 123/3 | 0.88 |
| Height (cm) | 153.1 [134–178] | 152.4 [134–170] | 0.31 |
| Body weight (kg) | 56.1 [32–93] | 54.1 [35–80] | 0.1 |
| BMI (kg/m2) | 24.0 [13.5–44.2] | 23.2 [16.1–34.6] | 0.18 |
| Operation method, SLNB/ALND | 132/94 | 101/25 | <0.0001 |
Data are presented as mean [range] or number. BMI, body mass index; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; MPH, microporous polysaccharide hemospheres.
Table 2
| Characteristics | Control (n=132) | MPH (n=101) | P value |
|---|---|---|---|
| Age (years) | 66.7 [35–89] | 69.2 [36–89] | 0.14 |
| Height (cm) | 152 [134–170] | 152 [139–170] | >0.99 |
| Body weight (kg) | 56.6 [32–93] | 54.3 [35–80] | 0.11 |
| BMI (kg/m2) | 24.4 [13.5–44.2] | 23.3 [16.1–34.6] | 0.08 |
| Intraoperative blood loss (mL) | 26 [0–235] | 29 [0–128] | 0.43 |
| Number of dissected lymph nodes | 2.4 [0–15] | 2.1 [0–11] | 0.3 |
| Drain placement period (days) | 5.8 [3–14] | 4.7 [3–13] | 0.0003 |
| Total drainage volume (mL) | 321 [27–1,761] | 254 [68–1,010] | 0.03 |
| Postoperative complications, present/absent/NA* | 31/81/20 | 21/67/13 | 0.54 |
Data are presented as mean [range] or number. *, not available because some patients were transferred to other hospitals after surgery. BMI, body mass index; NA, not available; MPH, microporous polysaccharide hemospheres.
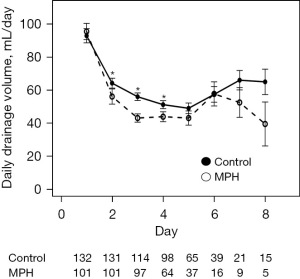
Table 3
| Characteristics | Control (n=94) | MPH (n=25) | P value |
|---|---|---|---|
| Age (years) | 61 [29–87] | 68 [38–89] | 0.03 |
| Height (cm) | 154 [134–178] | 152 [134–166] | 0.2 |
| Body weight (kg) | 55 [36–88] | 53 [38–74] | 0.38 |
| BMI (kg/m2) | 23.3 [15.6–34.2] | 23.0 [17.3–28.7] | 0.75 |
| Intraoperative blood loss (mL) | 42 [0–244] | 55 [0–176] | 0.17 |
| Number of dissected lymph nodes | 17 [3–41] | 14 [8–22] | 0.12 |
| Drain placement period (days) | 9.8 [4–14] | 8.3 [3–14] | 0.038 |
| Total drainage volume (mL) | 925 [168–2,720] | 709 [156–1,874] | 0.064 |
| Post operative complications, present/absent/NA* | 39/43/12 | 8/15/2 | 0.28 |
Data are presented as mean [range] or number. *, not available because some patients were transferred to other hospitals after surgery. BMI, body mass index; NA, not available; MPH, microporous polysaccharide hemospheres.
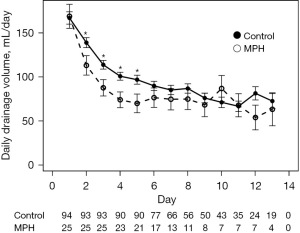
Table 4
| Variables | SLNB | ALND | |||||
|---|---|---|---|---|---|---|---|
| Control (n=132) | MPH (n=101) | P value | Control (n=94) | MPH (n=25) | P value | ||
| Any complications, present/absent/NA (n) | 31/81/20 | 21/67/13 | 0.54 | 39/43/12 | 8/15/2 | 0.28 | |
| Hemorrhage, present/absent (n) | 1/131 | 0/101 | 0.38 | 2/92 | 0/25 | 0.46 | |
| Infection, present/absent (n) | 2/130 | 0/101 | 0.21 | 6/88 | 0/25 | 0.2 | |
| Skin necrosis, present/absent (n) | 6/126 | 0/101 | 0.03 | 8/86 | 1/24 | 0.004 | |
| Seroma, present/absent/NA (n) | 28/84/20 | 17/81/3 | 0.18 | 32/50/12 | 7/16/2 | 0.45 | |
| Number of aspiration for seroma, mean | 1.9 | 3 | 0.07 | 2.8 | 2.3 | 0.56 | |
| Total amount of seroma (mL), mean | 145 | 221 | 0.33 | 292 | 297 | 0.98 | |
NA, not available; SLNB, sentinel lymph node biopsy; MPH, microporous polysaccharide hemospheres; ALND, axillary lymph node dissection.
Discussion
MPH is theoretically expected to reduce hemorrhage and serous exudate (1,3), while its clinical effectiveness remains controversial. Several randomized, controlled studies in various types of surgery, including breast surgery, could not establish the effectiveness of MPH (4-6), while other retrospective studies showed the effectiveness of MPH in cardiothoracic surgery and total knee arthroplasty (7,8). One reason for such a discrepancy may be the small study populations that were analyzed. Suarez-Kelly and colleagues could not show the effectiveness of MPH in mastectomy, but that study included only 50 patients who underwent various axillary manipulation techniques (4). In the current study, we analyzed a much larger population (352 patients) and showed the effectiveness of MPH in breast cancer surgery.
In our cohort, the incidence of postoperative complications was 28% (100 of 352 cases). Previous studies reported postoperative seroma incidence ranging from 0 to 35% in patients who underwent total mastectomy (4,9-11). Our outcomes are comparable with the previous data.
When we divided the patient population into two subgroups by surgical procedure (with and without ALND), MPH significantly decreased the drain placement period and the daily drainage volume in both subgroups, and the total drainage volume in the SLNB subgroup (Tables 2,3, Figures 1,2). In both the ALND and SLNB subgroups, the daily drainage volume in the MPH group reached a plateau and overlapped with that of the control group. This was attributed to the dropout of patients whose drainage output was small and whose tubes were removed. There was no difference between the MPH group and the control group in postoperative complications, except for skin necrosis. It is unclear why skin necrosis was less frequent in the MPH group. However, since the incidence of complications other than seroma was low, future studies with larger numbers of patients are needed.
Many studies have shown no effect of hemostatic agents, including MPH, fibrin glue, oxidized regenerated cellulose, polysaccharide hemostatic agents and local sclerosing agents, in breast cancer surgery (4,12-18). However, a systematic review concluded that the use of fibrin glue reduced the incidence of seroma, the postoperative drainage volume, and the duration of drainage (19). Thus, the efficacy of hemostatic agents remains controversial.
One disadvantage of using MPH is cost. One gram of MPH costs about $85 in Japan. The time required for use of MPH in surgery is less than one minute. Because the substance is derived from starch, there is no risk of allergy, and no other complications were observed in our experience.
This study has some limitations. First, it was a retrospective study, and some selection biases existed between groups. Age was higher in the MPH group than in the control group, especially in the ALND subgroup. Previous studies reported several risk factors that could contribute to drainage volume and seroma formation. Burak and colleagues reported risk factors for seroma formation after mastectomy and ALND as increased age, patient weight and other factors (20). In this study, the MPH group with elderly patients had smaller drainage volumes and a decreased incidence of seroma suggesting that the effectiveness of MPH was not affected by the selection bias of age. In addition, differences in surgeon experience and surgical equipment existed, because the timing of surgery was different in groups with and without MPH use. The detailed differences were not examined in this study. Second, the impact of MPH on number of hospitalization days was not examined, because it could be influenced by patient social background characteristics.
As a result, we showed that the use of MPH could decrease the drainage output and the number of drainage days. A disadvantage of using MPH is the increased cost of the surgical procedure, but the total cost effectiveness should be revealed with further study. For evaluation of the definitive clinical effectiveness of MPH in breast cancer surgery, a clinical trial with an appropriate number of cases is needed.
Conclusions
Use of MPH in mastectomy for patients with breast cancer was associated with decreased drainage output and fewer drainage days.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-297/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-297/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-297/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-297/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Kagoshima University Hospital (No. 220041-Epidemiology). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lewis KM, Atlee H, Mannone A, et al. Efficacy of hemostatic matrix and microporous polysaccharide hemospheres. J Surg Res 2015;193:825-30. [Crossref] [PubMed]
- Singh RK, Baumgartner B, Mantei JR, et al. Hemostatic Comparison of a Polysaccharide Powder and a Gelatin Powder. J Invest Surg 2019;32:393-401. [Crossref] [PubMed]
- Egeli T, Sevinç Aİ, Bora S, et al. Microporous polysaccharide hemospheres and seroma formation after mastectomy and axillary dissection in rats. Balkan Med J 2012;29:179-83. [Crossref] [PubMed]
- Suarez-Kelly LP, Pasley WH, Clayton EJ, et al. Effect of topical microporous polysaccharide hemospheres on the duration and amount of fluid drainage following mastectomy: a prospective randomized clinical trial. BMC Cancer 2019;19:99. [Crossref] [PubMed]
- Kunduz E, Aysan E, İdiz UO, et al. Evaluation of local hemostatic effect of microporous polysaccharide hemospheres products in thyroid surgery: a prospective randomized controlled study. Turk J Surg 2019;35:49-53. [Crossref] [PubMed]
- Antisdel JL, Matijasec JL, Ting JY, et al. Microporous polysaccharide hemospheres do not increase synechiae after sinus surgery: randomized controlled study. Am J Rhinol Allergy 2011;25:268-71. [Crossref] [PubMed]
- Bruckner BA, Blau LN, Rodriguez L, et al. Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures. J Cardiothorac Surg 2014;9:134. [Crossref] [PubMed]
- Gleason S, Mehl D, Payne W, et al. Microporous polysaccharide hemosphere efficacy and safety in primary total knee arthroplasty. J Orthop 2019;16:19-24. [Crossref] [PubMed]
- Andeweg CS, Schriek MJ, Heisterkamp J, et al. Seroma formation in two cohorts after axillary lymph node dissection in breast cancer surgery: does timing of drain removal matter? Breast J 2011;17:359-64. [Crossref] [PubMed]
- Marla S, Stallard S. Systematic review of day surgery for breast cancer. Int J Surg 2009;7:318-23. [Crossref] [PubMed]
- Barton A, Blitz M, Callahan D, et al. Early removal of postmastectomy drains is not beneficial: results from a halted randomized controlled trial. Am J Surg 2006;191:652-6. [Crossref] [PubMed]
- Carless PA, Henry DA. Systematic review and meta-analysis of the use of fibrin sealant to prevent seroma formation after breast cancer surgery. Br J Surg 2006;93:810-9. [Crossref] [PubMed]
- Bloom JA, Foroutanjazi S, Erlichman Z, et al. The Use of Hemostatic Agents to Decrease Bleeding Complications in Breast Cancer Surgery. Am Surg 2023;89:395-400. [Crossref] [PubMed]
- Weber WP, Tausch C, Hayoz S, et al. Impact of a Surgical Sealing Patch on Lymphatic Drainage After Axillary Dissection for Breast Cancer: The SAKK 23/13 Multicenter Randomized Phase III Trial. Ann Surg Oncol 2018;25:2632-40. [Crossref] [PubMed]
- van Bastelaar J, Granzier R, van Roozendaal LM, et al. A multi-center, double blind randomized controlled trial evaluating flap fixation after mastectomy using sutures or tissue glue versus conventional closure: protocol for the Seroma reduction After Mastectomy (SAM) trial. BMC Cancer 2018;18:830. [Crossref] [PubMed]
- Nam KH, Lee JH, Chung YS, et al. The efficacy of oxidized regenerated cellulose (SurgiGuard®) in breast cancer patients who undergo total mastectomy with node surgery: A prospective randomized study in 94 patients. PLoS One 2022;17:e0267694. [Crossref] [PubMed]
- Falcone V, Krotka P, Deutschmann C, et al. Use of polysaccharide hemostatic agent (HaemoCer™) in breast cancer surgery to reduce postoperative complications: A randomised controlled trial. Int Wound J 2023;20:925-34. [Crossref] [PubMed]
- Khater A, Hassan A, Farouk O, et al. Evaluation of Topical Sclerosant Agents for Minimization of Postmastectomy Seroma: A Placebo-Controlled, Double-Blind, Randomized Trial. Eur J Breast Health 2023;19:134-9. [Crossref] [PubMed]
- Gasparri ML, Ruscito I, Bolla D, et al. The Efficacy of Fibrin Sealant Patches in Reducing the Incidence of Lymphatic Morbidity After Radical Lymphadenectomy: A Meta-Analysis. Int J Gynecol Cancer 2017;27:1283-92. [Crossref] [PubMed]
- Burak WE Jr, Goodman PS, Young DC, et al. Seroma formation following axillary dissection for breast cancer: risk factors and lack of influence of bovine thrombin. J Surg Oncol 1997;64:27-31. [Crossref] [PubMed]


