
Evolution of breast conserving surgery—current implementation of oncoplastic techniques in breast conserving surgery: a literature review
Introduction
Breast cancer is the most common cancer among women worldwide, with one out of seven women suffering a breast cancer in their lifetime (1). Breast conserving surgery (BCS) was adopted three decades backward, as trials like NSABP B-06, EORTC and the Milan trials demonstrated similar survival and recurrence rates for BCS compared to simple mastectomy and Halsted mastectomy procedures in early breast cancer (2-4). Even some authors suggest recently better survival outcomes comparing BCS versus mastectomies irrespective of reconstruction techniques (5). Additionally, it’s become clear that, if possible, BCS should be performed, since better quality of life (QoL), psychological well-being and aesthetic outcomes have been reported comparing to mastectomy (6,7).
At a similar onset of the standard BCS (S-BCS), the term oncoplastic BCS (O-BCS) was firstly used, at the “Santa Fe Symposium on Breast Surgery and Body Contouring” in 1993 (8), but was not until 2006 in the Milan conference when the aims of the oncoplastic were defined, being these the complete excision of the tumor with free margins, minimal aesthetic compromise and simultaneity of the breast tissue reshaping if needed. Since then, interest in oncoplastic surgery within the scientific community has grown exponentially, leading to a “change of paradigm” in breast surgery in 2014 (9). This shift is reflected in recent international guidelines, such as National Comprehensive Cancer Network (NCCN) recommendations (10). This represents a fundamental change in how this type of surgery is approached and conducted, involving a departure from traditional practices, approaches or perceptions. We reference the advancement of techniques, comprehension of outcomes, and the integration of oncoplastic surgery into the broader context of breast cancer treatment. This transformation could result from technological progress, emerging scientific evidence and an approach centered on patients’ well-being and QoL. Despite this increasing interest, oncoplastic techniques are not fully implemented in many breast cancer units world-wide yet.
The review aimed to provide a comprehensive overview of recent findings and perspectives in the field, highlighting the benefits and risks that O-BCS may offer. This contributes to the ongoing debate on the role and implementation of oncoplastic surgery in breast cancer units. We present this article in accordance with the Narrative Review reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-454/rc).
Methods
A literature review was conducted to identify articles related to oncoplastic surgery in breast cancer published in English up to March 2023 (Table 1). The focus was on publications that provided insights into the application, oncological and QoL outcomes, and challenges of oncoplastic surgery in the context of breast cancer treatment.
Table 1
| Items | Specification |
|---|---|
| Date of search | April 20th, 2023 |
| Databases and other sources searched | PubMed-MEDLINE, Embase and Cochrane Database |
| Search terms used | “Oncoplastic surgery”, “breast conservative surgery” |
| Timeframe | January 1985 to March 2023 |
| Inclusion criteria | Reviews, systematic reviews, prospective and retrospective studies, case reports published in English |
| Selection process | Selection was independently carried out by the authors |
Oncologic outcomes
Local recurrence (LR), disease-free survival (DFS) and overall survival (OS)
In the context of O-BCS achieving good outcomes in terms of LR, DFS, and OS is essential. Reducing the risk of LR while maintaining a patient’s OS and DFS are indicative of successful breast cancer management. The latest Cochrane review (11) published in 2021, which includes 78 non-randomized cohort studies evaluating 178,813 women, indicates that when comparing O-BCS to S-BCS, there may be little or no difference in terms of LR [hazard ratio (HR) 0.90, 95% confidence interval (CI): 0.61–1.34] and DFS (HR 1.06, 95% CI: 0.89–1.26). In comparison to mastectomy alone, O-BCS may lead to an increase in LR-free survival (HR 0.55, 95% CI: 0.34–0.91).
When compared to mastectomy with reconstruction, O-BCS may show little or no difference in LR-free survival (HR 1.37, 95% CI: 0.72–2.62) or DFS (HR 0.45, 95% CI: 0.09–2.22).
Despite oncoplastic procedures seems to offer comparable oncological results, it should be highlighted that most studies in oncoplasty are retrospective in nature, and there is still a lack of prospective randomized multicenter clinical trials in the literature. In addition, it should be considered that O-BCS comprises many techniques and each individual breast cancer patient does not have a unique surgical solution, thus there is great heterogeneity in oncoplastic techniques among studies when evaluating oncoplastic safety, since both reduction mammoplasties and volume replacement with local flaps are usually included in their analysis. It is challenging to conduct a randomized controlled trial (RCT) comparing O-BCS to mastectomy or S-BCS, given ethical considerations, patient preferences, clinical variability, and changing contexts in medical practice. Also, it is plausible that patients opting for reconstruction may have smaller and less aggressive tumors compared to mastectomy without reconstruction, influencing the outcomes and making them more similar to those achieved with O-BCS.
Summarizing, the evidence is very uncertain regarding oncological outcomes following O-BCS, although has not been shown to be inferior.
Hence, current data supports the use of O-BCS in patients with breast cancer but better designed studies are needed to provide more robust data on its safety.
Margin status, lesion localization and tumor bed
In BCS, achieving clear margins is a critical aspect of the surgical procedure. S-BCS aims to remove the tumor with a margin of healthy tissue but may be more conservative in terms of breast tissue removal. O-BCS involves the simultaneous removal of the tumor while reshaping the breast. It often allows for a more extensive resection while preserving a satisfactory cosmetic outcome. The ability to achieve clear margins in O-BCS may be influenced by the surgeon’s expertise in breast reshaping techniques. In most cases, the risk of positive margins can be lower due to the wider resections and the flexibility provided by these techniques. Losken et al. (12) showed a lower incidence of positive margins in oncoplastic surgery compared to S-BCS (12.2% versus 20.6%). Positive margin rate reported in oncoplastic studies ranges from 10.9% to 18.9% (13-15). In a systematic review (11), it was concluded that O-BCS may reduce the rate of re-excisions needed for oncological resection [risk ratio (RR) 0.76, 95% CI: 0.69–0.85], but the evidence is very uncertain.
The pre-surgical localization of lesions is a crucial point, and if necessary, employing multiple techniques or multiple markers to delineate the area for resection. Wire-guided localization (WGL) is the most common used localization method, and it is considered the standard localization method of non-palpable breast lesions. Notwithstanding, newer technologies have emerged that enable the localization of lesions with a similar detection rate and clear margins, enhancing the experiences for both the surgeon and the patient. These include radio-guided occult lesions localization (ROLL), intraoperative ultrasound (IOUS), seeds [Radioactive Seed, MagSeed® (Endomagnetics Inc., Cambridge, UK), SAVI Scout® (Merit Medical, South Jordan UT, previously Cianna Medical, Aliso Viejo, CA, USA)], among others.
In this context, the ongoing EUBREAST MELODY study aims to assess different imaging-guided localization methods in terms of oncological safety, patient-reported outcomes, and satisfaction levels among surgeons and radiologists. The target accrual is 7,416 patients, with enrollment starting in January 2023. The study will be conducted across 20 countries (16).
When facing O-BCS accurate localization of tumor bed, detailed specimen orientation and clear marking of lumpectomy cavity are important factors not only for surgery success, but also for guiding further procedures such as margin re-excision when needed and radiotherapy planning (17). In order to reduce positive margins rates during oncoplastic procedures some groups propose several options to assess intraoperatively margin status, such as routine margin shaving or intraoperative specimen radiography and gross pathological evaluation to guide the need for further tissue resection during index surgery (18-20). The most well-established methods for margin assessment include gross inspection, frozen section analysis (FSA), and imprint cytology (IC). According to one systematic review, FSA and IC could reduce reoperation rates from 35% to 10% and 11%, respectively (21).
Radiological methods have shown promising results, with numerous studies unanimously demonstrating the excellence of IOUS in achieving negative margins, reducing resection tissue volume, and improving overall aesthetic results and patient satisfaction (22).
Regarding the use of mammography, the reported sensitivity of specimen mammography for intraoperative margin assessment ranged from 20.6% to 45.45% (23). According to the authors, mammography would be highly useful in cases that radiologically present as microcalcifications.
An emerging trend involves the participation of artificial intelligence (AI) during image identification. Novel techniques provide alternative approaches to evaluating margins during surgery and include radiofrequency spectroscopy, bio-impedance spectroscopy, and optical coherence tomography (OCT). There are also preliminary studies involving the use of drugs to modify and make lesions visible, such as studies including EC17 and trastuzumab, or 18F-fluorodeoxyglucose (18F-FDG) used for specimen positron emission tomography-computed tomography (PET-CT).
Nevertheless, BCS for ductal carcinoma in situ (DCIS) and BCS after neo-adjuvant chemotherapy pose significant challenges in achieving negative margins.
“Negative margins” is currently considered as no ink on the tumor when we are referring to infiltrating breast carcinoma, as indicated by the NCCN guidelines (10). However, distinctions arise in cases of DCIS, where margins of at least 2 mm are linked to a decreased risk of ipsilateral breast tumor recurrence (24). While oncoplastic level II resections in high-risk breast cancer patients enhance margin width, they do not correlate with lower rates of LR. Interestingly, the use of oncoplastic level II techniques significantly reduces the number of re-excisions attributed to R1 (25).
In De la Cruz’s systematic review (26), the rate of positive margins in oncoplastic surgery varied widely (0–39.7%), given that the assessment of positive margins is highly heterogeneous. Eleven studies reported specific margins for 1,455 patients. Among these patients, 143 (9.8%) were classified as having positive margins, of which 113 (7.8%) had ink on the tumor.
The problem lies in cases of oncoplastic surgery with involved margins and the oncological safety of margin re-excision. According to the authors, we believe that margin re-excision is feasible even if there has been glandular mobilization. To achieve this, it’s important to mark both the tumor bed and the surgical field with clips, ensure good communication between the pathologist and the surgeon, and ensure concordance between imaging results and pathological findings. It won’t be the same a margin in focal contact as it would be for several involved margins or multifocal/multicentric lesions. If possible, it is advisable that the same surgeon performs both surgeries. One must be realistic when considering the possibility of margin re-excision to avoid false reassurance.
In some cases, patients with positive margins after O-BCS will proceed to mastectomy. Nevertheless, there is a great discrepancy in mastectomy conversion rates after upfront O-BCS in the literature for involved margins, ranging from 12.5% to 100% (15,27-31). Despite this fact, mastectomy is not always necessary when managing a positive margin after O-BCS. In a retrospective study where 649 patients underwent oncoplastic Wise pattern reduction, 95% were successfully managed with margin re-excision while maintaining breast-conserving therapy. There was only one in-breast recurrence in this case series (32). The use of magnetic resonance imaging (MRI), which enhances lesion detection sensitivity, could potentially increase the mastectomy rate. Additionally, it has not been demonstrated that the use of MRI increases the risk of involved margins.
In summary, despite great volume displacement resulting during oncoplastic procedures, re-excision for margin clearance is possible as long as margin involvement is focal in pathological specimen. Otherwise, when facing multiple margins affection ensuring new clear margins would be challenging and a mastectomy should be offered.
Mastectomy reduction rate: extreme oncoplasty [neoadjuvant chemotherapy (NAC) and DCIS]
Oncoplastic surgery is extending the role of BCS to an increasing number of patients who are candidates for mastectomy. This new approach includes patients with locally advanced breast cancer tumors larger than 5 cm at presentation, multifocal and multicentric disease and intraductal carcinoma with extensive involvement. Silverstein et al. was the first to introduce the term extreme oncoplastic (EO) to describe breast cancer patient candidates for BCS for which most physicians would performs a mastectomy (33). Trying to get a more adjusted definition of EO-BCS, we must push Clough et al.’s classification beyond level 2, were breast volume excision greater than 50% or skin replacement would be needed to achieve free margins tumor excision (34). In these cases, we should consider volume displacement techniques to reconstruct partial breast defect using mammoplasty techniques including local glandular tissue advancement flaps, mastopexy, and reduction mammoplasty or volume replacement procedures, including autologous flaps designed to reconstruct a new breast after resection, such as chest wall perforator flaps (CWPFs), among which are the lateral intercostal artery perforator (LICAP), lateral thoracic artery perforator (LTAP), a combined flap, and anterior intercostal artery perforator/medial intercostal artery perforator (AICAP)/(MICAP).
These extreme procedures should also be considered even in the neoadjuvant setting for tumors that did not shrink optimally after NAC or in the presence of extensive intraductal disease. NAC was initially introduced for patients with large breast cancer to downsize the tumor in an attempt to allow breast conservation for patients who would have been treated by mastectomy. Van la Parra et al. (35), showed that EO-BCS can further extend the indications for breast conservation after NAC, providing equal local control to those tumors that did respond optimally and underwent S-BCS, and similar to smaller cancers that did not undergo NAC.
Because of the great amount of breast resection carried out during EO procedures, immediate or delayed breast symmetrization should be offered when considering patients for O-BCS, especially in EO procedures definition. The ideal timing for symmetrization is not clear and remains controversial. Some authors argue that it should occur after index breast surgery and the administration of adjuvant radiotherapy, as longitudinal changes due to radiotherapy can affect the final outcome. However, predicting these changes can be challenging (36).
Other authors (37,38) believe that contralateral symmetrization could be performed at the time of O-BCS in carefully selected patients without significantly increasing the risk of complications or delaying adjuvant radiation therapy. Also delayed symmetrization in BCS resulted in an additional cost when compared with immediate bilateral mammoplasty. In this context, it is relevant to address the availability of operating theaters, as this surgical intervention aims to achieve aesthetic symmetry. In some countries, there is a significant limitation in terms of access to operating theaters and adequately trained medical staff, which can prevent or hinder the execution of contralateral symmetrization procedures.
EO is an excellent alternative to mastectomy since locally advanced tumors are most of breast cancer candidates for oncoplastic procedures requiring radiation therapy anyway. Radiotherapy after conservative surgery will offer kinder results than mastectomy with implant reconstruction followed by mastectomy chest wall irradiation in term of QoL, cosmetic results and healthcare costs (39,40).
Complications
The Clavien Dindo Classification assesses the severity grade of postoperative complications in breast surgery on a scale from 1 to 5. Grades 1–2 represent minor complications (requiring no treatment or only pharmacological treatment), Grades 3–4 signify major morbidity (requiring surgical treatment and involving life-threatening complications), and Grade 5 is associated with postoperative death (41).
Complication rates for oncoplastic procedures reported in most studies are relatively high (range, 16–30%) (14,20,29,42) although there are also a few studies in large populations reporting lower complications rates (8–10%) (13,15). O-BCS and EO inherently involve greater technical complexity and are associated with glandular tissue mobilization that may entail a higher risk of fat necrosis and complications compared to S-BCS (43). According to Nizet et al., size resection was the only factor associated with postoperative complications, confirming that complexity of O-BCS is linked to postoperative complications risk (44).
Fat necrosis and wound dehiscence, ranging from 0.9% to 6% (45,46), are uncommon yet challenging complications of oncoplastic procedures. It is important to emphasize that while these issues are indeed associated with technical flaws, patient-related risk factors have been identified as significant influences on wound healing, underscoring the necessity for careful patient selection. This consideration aligns with findings demonstrating that high-volume oncoplastic BCS is an independent risk factor for delayed wound healing (47,48).
Extreme fine dissection of glandular flaps and excessive suture tension in wound predispose to their appearance. Thus, adjuvant treatments administrations will be delayed until wound closure is settled, which may result in prognosis impairment. On the other hand, although fat necrosis is usually asymptomatic it is and evolving complication enhanced by radiation therapy (45,46).
Cosmetic sequelae (CS) should also be considered when analyzing oncoplastic results, since it usually arises during the first 5 years after surgery and affect up to 17% of oncoplastic procedures. Acea-Nebril et al. proved in a multivariate analysis that CS were significant related to complexity of oncoplastic procedure [odds ratio (OR) 2.605; 95% CI: 1.623–4.181; P<0.01] and clinical postoperative complications (OR 4.626; 95% CI: 2.719–7.868; P<0.01), especially fat necrosis and hematoma (49).
Finally, increase in postoperative complication rate derived from oncoplastic procedures is an issue of great concern, because it may cause delay in adjuvant treatments administration. The Cochrane review by Nanda et al. suggests that the time to adjuvant therapy may be increased, specifically in the case of adjuvant radiotherapy, when utilizing O-BCS as opposed to S-BCS. This potential extension in time could be attributed to delays arising from complications. The delay in adjuvant radiotherapy is estimated to range between 7.21 and 12.1 days, which could hold clinical significance (11).
Skilled and trained surgeons in oncoplastic techniques are needed in present and future breast cancer units in order to reduce technical failures. Accurate selection of both, patients who are candidate for O-BCS and selective mammoplasty techniques adapted to each individual situation is essential to improve oncoplastic complications rate (34).
QoL outcomes
QoL is a multidimensional concept with challenging evaluating issues. QoL usually includes traditional outcomes such as survival, efficacy, and safety, but they do not provide a complete picture, thus assessing well-being emotional perception component, patients’ preferences, goals, and personal satisfaction are also crucial. These aspects are related to different aspects of life, such as physical and mental health, social relationships, and economic status.
O-BCS is often considered to have a positive impact on patient’s QoL outcomes, as it can provide better cosmetic results and a lower risk of sequelae compared to mastectomy (50). However, the specific impact can vary for each individual and may depend on various factors such as tumor size and location, patient’s overall health, and type of treatment received after surgery. Evidence is required to assess whether high-volume O-BCS, which entails a heightened risk of complications but potentially a lower rate of re-excisions, may impact QoL.
Patient-reported outcome measures (PROMs) are assessments of health status, function, or symptoms directly reported by patients, rather than observed or recorded by clinicians. These measures provide valuable information on patient’s perspective and can complement traditional clinical measures. PROMs have become increasingly important in breast surgery evaluation, as they allow patients to provide feedback on their outcomes and help to identify areas for improvement in care delivery. One of the most widely used tools to evaluated QoL is BREAST-Q (51), a validated and specific test, translated into many languages, which includes physical, psychosocial, sexual and satisfaction questions. BREAST-Q has become the gold standard PROMs instrument for breast surgery.
There are other PROMs used to assess outcomes in breast surgery, including European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30, the EORTC QLQ-BR23 and QLQ-BR45 questionnaires, The Functional Assessment of Cancer Therapy-Breast (FACT-B) or MD Anderson Symptom Inventory module specific to breast cancer (MDASI-Br) (52-55).
Currently, the COSMAM study is being conducted at a single-center in the Netherlands. This prospective study aims to evaluate the QoL and cosmetic outcomes in patients undergoing standard lumpectomy versus level I or II O-BCS (56).
Aesthetic results
Achieving good cosmetic result is one of the factors which is proportional and directly linked to QoL (57). Evaluation of aesthetic results can be subjective, based mainly on patient’s self-assessment or evaluation by a single or panel of observers. It can also be objective, using tools like Breast Symmetry Index (BSI) or Breast Cancer Conservation Treatment cosmetic results software (BCCT.core) which measure symmetry and proportion in postoperative photographs (58,59).
Three-dimensional surface imaging (3D-SI) is being marketed as a tool in aesthetic breast surgery, and it has recently also been studied in the objective evaluation of cosmetic outcome of oncological procedures and have the potential to assist in pre-operative planning (60,61). Efforts are ongoing to develop objective measures for this subjective concept.
When comparing O-BCS to standard breast surgical techniques, O-BCS volume displacement procedures had significantly better aesthetic outcomes than conventional BCS, either if objective methods (breast retraction assessment) and subjective methods (panel assessment) are used in evaluation, as well as when body image questionnaires are available (62).
It is crucial to note that tumor size and location play a significant role. For larger primary tumors in cosmetically sensitive zones of the breast, O-BCS is likely to result in significantly improved aesthetic outcomes when compared to S-BCS.
In two studies including over 120 patients with unilateral O-BCS, BREAST-Q “Satisfaction with their breast” median score was reported between 65–74/100, and factors associated with a score below median value were axillary clearance (OR 2.46, 95% CI: 1.09–5.56), NAC (OR 3.26, 95% CI: 1.15–9.24), and low breast density (OR 2.32, 95% CI: 1.02–5.29). It is remarkable that only 11% of these patients were interested in contralateral surgery (63,64). Similar results regarding breast symmetry where reported by de Oliveira-Junior et al. in a series where contralateral surgery for symmetrization was not associated with high patient satisfaction (65).
When compared with mastectomy, a literature review showed significantly higher scores in BREAST-Q questionnaire in O-BCS, regardless of the type of the reconstruction performed after mastectomy (66).
When analyzing QoL results according to oncoplastic technique used, volume displacement techniques reported significantly higher scores for “physical well-being of the chest” than patients who underwent volume replacement. Also, patients without complications had significantly higher scores in “satisfaction with the breast” and “satisfaction with information about the surgery” domains compared to patients with complications (64).
Sexual well-being
Breast cancer survivors have the highest rates of lost disability-adjusted life years (DALYs) among all types of cancer and often experience high rates of sexual dysfunction (SD) as well, which can persist for years and significantly impact their QoL (67). SD rates among breast cancer survivors can range from 60–90% (68-72). It is a multifactorial entity severely influenced by the secondary effects of treatments and the psychological impact of presenting the disease itself. Breast cancer survivors often report various symptoms of SD, including difficulties with arousal or excitation, decreased sexual desire, insufficient lubrication, and penetration pain. These symptoms can have a profound impact on sexual function.
Surgery causes a direct disruption in body image. This alteration is magnified by the fact that breasts, apart from being one of the key erogenous parts of the female body, are considered to be symbols of sexuality and sexual identity. The section on sexual well-being in the BREAST-Q questionnaire has been reported to receive lower scores compared to other sections in many studies.
Some studies suggest that BCS with radiotherapy can lead to clinically meaningful improvements in psychosocial and sexual well-being for women with early breast cancer, compared to those who underwent mastectomy with reconstruction (73).
A systematic review found that women who underwent O-BCS had better sexual well-being and residual skin sensitivity compared to those who underwent mastectomy, despite the type of reconstruction (74). These findings suggest that preserving the breast tissue and improving breast appearance through oncoplastic techniques can have a positive impact on sexual well-being for breast cancer survivors (75). However, it is important to note that individual experiences may vary and additional support may still be needed.
Psychological well-being
There are two components as main cornerstones of psychological well-being: the cognitive component, which refers to global judgments about life satisfaction; and the affective component, which refers to feelings about life experiences and the roles a person holds. Both components are important for overall psychological well-being and contribute to successful social functioning (76).
Patient’s perception of preoperative information and the opportunity to participate in decision-making are crucial factors in determining satisfaction with diagnostic-therapeutic process. Patients who feel involved and well-informed during the process are more likely to have positive outcomes and be satisfied with the results. This is why it is essential for healthcare providers to settle effective and clear communicative pathways with their patients, listen to their concerns and preferences, and involve them in the decision-making process as much as possible (77).
Surgical removal of gross part of breast tissue can result in visible scars, deformities, or asymmetry, which can have a negative impact on mental health, including anxiety, depression, body image issues, and difficulties with sexual intimacy. Postoperative recovery and its sequelae are involved in QoL, however other subjective factors such as the aesthetic result and changes in physical appearance play an important role for a large part of the patients. Also, undergoing multiple interventions for cancer treatment may have a significant impact on a person’s daily life and overall health perception. It can lead to chronic distress and make it difficult for them to return to their normal routine (78).
Research in the field of literature indicates that patients tend to experience improved physical and psychological health when oncoplastic surgery techniques are employed, as opposed to undergoing mastectomy with or without reconstruction (79). Additionally, the average psychosocial well-being score in the BREAST-Q questionnaire is notably higher in patients who undergo O-BCS when compared to those who opt for simple S-BCS alone (41.94±5.78 versus 38.02±7.21; with a statistically significant P value of <0.0001) (80).
Current implementation of oncoplasty
Standardization and evaluation of oncoplastic techniques
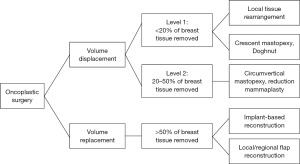
Several O-BCS classifications have been proposed to create homogeneity, decrease complexity, and form a basic lexicon for patients, surgeons, trainees, and educators for worldwide standardization. Currently, the most used classification divides the techniques into two groups: volume displacement procedures and volume replacement interventions.
Clough et al. (34) classified oncoplastic surgical techniques based on the breast volume to be resected and the quadrant of the tumor located serving to standardize O-BCS to adopt in routine clinical practice. They included only volume displacement techniques denoted up to 20% of the breast volume to be resected as level I and 20–50% of the volume to be resected as level II techniques.
In 2019, the American Society of Breast Surgeons (ASBrS) performed a comprehensive literature search and created a consensus definition and classification based on 30 articles defining oncoplastic surgery (81), using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1).
When considering cancer surgery outcomes, case-adjusted improvements in long-term survival probably represent the best measure of performance. But today, demand in breast surgery has increased, being essential the aesthetic results and QoL outcomes.
Future of quality improvement and standardization is only possible by conceptualization of value through quality indicators (82). It is currently recommended to carry out systematically questionnaires that can serve as tools to assess QoL and cosmetic results, as well as a registry of morbidity, complications and post-surgical sequelae in prospective trials. It is also essential to take images before surgery and during follow-up to acquire scientific evidence and evaluate aesthetic results of oncoplastic procedures. Photographic documentation of patients before and after surgery is an important standard for clinical routine practice, as recommended by the panel in the first international consensus conference on standardization of oncoplastic surgery in 2017 (83).
Aesthetic results in breast surgery are dynamics, influenced by physical changes such as weight change or aging, as well as others factors derived from treatments, such as radiotherapy. Times and periodicity for iconography acquisition and storage should be standardized, requiring a baseline image, before radiotherapy, 1 year after radiotherapy and 5 and 10 years after surgery (84). Unfortunately, the major issue about O-BCS outcomes is the absence of standardized quantitative evaluation measures to permit comparative research and to access high level of evidence which is a must to create applicable guidelines.
A review published in 2021 by a panel of experts utilized the GRADE system to analyze published data. Despite certain areas of controversy, approximately one-third (36%) of the panel members strongly recommended O-BCS (85).
Patient expectations play a crucial role in the overall success and satisfaction with any medical procedure, particularly in the field of breast surgery. In the absence of clear communication and alignment of expectations between patients and surgeons, there is a growing challenge leading to increased rates of litigation related to breast surgery in various countries. Addressing and managing patient expectations should be considered a key component in the comprehensive evaluation and standardization of oncoplastic techniques to ensure not only medical success but also patient contentment and reduced legal repercussions.
Learning curve in oncoplastic techniques and training
Historically, breast surgery was quite simple, all women were treated with mastectomy and axillary clearance without reconstruction, and it was performed by gynecologist and general surgeons who had finished their residency training programs with mixed contents. Modern breast surgery is now highly complex and such limited training is not adequate for actual standard of care.
When learning a new procedure, performance tends to improve with experience, and graphically plotting performance against experience produces a learning curve. The origins of this concept derive from aviation and industry, but it has been transferred to different areas of medical practice (86).
Trainees have to explore their learning process. The use of simulation and virtual reality can offer several advantages, such as providing a safe and controlled environment for surgeons to practice and refine their skills. These simulations can help train and educate medical professionals without exposing patients to unnecessary risks. It’s essential for researchers and practitioners in the field of breast surgery to consider and incorporate these innovative training tools and methodologies into their practice to enhance surgical skills and patient safety.
The advantage of having an organized breast unit with trained professionals and in training in oncological and reconstructive surgery techniques made possible to develop these procedures and obtain good results. The role of the breast surgeon mainly consists in proposing the optimal surgical treatment without compromising further adjuvant treatments. Plastic surgeons may be unavailable or not involved in breast cancer management, so it is mandatory that breast surgeons be trained and skilled in O-BCS techniques to provide the optimal quality of care (87).
It is important to point out that in all new surgical techniques the key relies on the cases needed to learn how to do the procedure and how it will be evaluated. Defining a number of procedures that are needed to reach a certain level of safety can be helpful for educational purposes. During surgical training, new skills and competences need to be acquired safely without compromising patient safety. Once the procedure is successfully performed then it is necessary to identify quality measures that evaluate outcomes and opportunities to improve the technique with appropriate feedback. The constant maintenance of the learning curve is necessary, especially in oncoplastic techniques of level 2 and 3 which represent a higher level of complexity.
Currently we do not have enough evidence about learning curve of O-BCS, but it has been studied in other surgical techniques of breast surgery that can guide us about the process. In a prospective study where learning curve of IOUS in BCS was evaluated, it was concluded that 11 cases were sufficient to acquire skills to perform the technique (88). Krekel et al. establish that the learning curve for this type of surgery would be two cases to obtain the basic concepts and skills and eight procedures to perform autonomously (89). A systematic review that included 29 studies focused on the learning curve of plastic surgery (including mastectomy, non-free flap and free flap reconstruction) did not allow pooling of the data because of heterogeneity, but improvement was demonstrated in operation time, success and complication rate with surgeon experience, and the plateau of the learning curve was reached after 45 to 100 cases (90). About endoscopic total mastectomy, it is described a plateau at 30 to 50 endoscopic total mastectomy procedures (91). Other authors reported learning curves in time, which are more prolonged, such as 8–12 years of experience on mammoplasty (92,93).
Despite these publications, the medical community has been moving away from using the number of repetitions or cases as the sole benchmark for proficiency and competence. Instead, there is a growing emphasis on establishing objective, expert-derived benchmarks that are based on a deeper understanding of the skills and competencies required in a particular medical procedure.
In USA breast surgery is a subspecialty with available fellowships. However, in Europe O-BCS techniques are performed by gynecologists, general or plastic surgeons who became breast surgeons after adequate training and experience, but at present is not a recognized subspecialty. To overcome this regulation difficulty, the European Breast Surgical Oncology (BRESO) initiated a pan-European curriculum for completely trained breast surgeons and proposes that all surgeons practicing in Europe should be certified, by means of undertaking high level training either within their residency (if available) or by means of approved specialist fellowships, which includes O-BCS (94). The Association of Breast Surgery (2) in the UK is a professional organization dedicated to promoting the highest standards in breast surgery. They provide education, training, and support for healthcare professionals involved in breast surgery.
Limitations
The literature included diverse study designs, ranging from case reports to prospective studies, leading to variability in the level of evidence and potential biases. Several studies had relatively small sample sizes, which may limit the generalizability of findings and the ability to detect significant differences or trends. The absence of RCTs in some areas of oncoplastic surgery limits the ability to establish causal relationships and ascertain the true effectiveness of specific interventions.
Future research should prioritize prospective studies with extended follow-up periods to better understand the long-term oncological and cosmetic outcomes of oncoplastic surgery. Research should aim for standardized reporting of outcomes and methodologies, facilitating more robust meta-analyses and systematic reviews.
Conclusions
O-BCS should be offered as a possible therapeutic option in every breast cancer unit world-wide, since evidence support its oncological safety and reports better QoL and well-being compared to S-BCS and mastectomy. In order to improve surgical results and avoid complications derived from increasingly complex oncoplastic procedures, only skilled and trained surgeons should be allowed to perform type 2 or 3 O-BCS. Simultaneously, it is breast surgeons’ responsibility to deal with scientific societies to finally certify continuing educational training in oncoplastic techniques.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-454/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-454/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-454/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- van Dongen JA, Bartelink H, Fentiman IS, et al. Factors influencing local relapse and survival and results of salvage treatment after breast-conserving therapy in operable breast cancer: EORTC trial 10801, breast conservation compared with mastectomy in TNM stage I and II breast cancer. Eur J Cancer 1992;28A:801-5. [Crossref] [PubMed]
- Veronesi U, Salvadori B, Luini A, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer 1995;31A:1574-9. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- de Boniface J, Szulkin R, Johansson ALV. Survival After Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48 986 Women. JAMA Surg 2021;156:628-37. [Crossref] [PubMed]
- Rosenberg SM, Dominici LS, Gelber S, et al. Association of Breast Cancer Surgery With Quality of Life and Psychosocial Well-being in Young Breast Cancer Survivors. JAMA Surg 2020;155:1035-42. [Crossref] [PubMed]
- Dominici L, Hu J, Zheng Y, et al. Association of Local Therapy With Quality-of-Life Outcomes in Young Women With Breast Cancer. JAMA Surg 2021;156:e213758. [Crossref] [PubMed]
- Audretsch W, Rezai M, Kolotas C. Oncoplastic surgery in breast conserving therapy and flap supported operability. Annual Symposium on Breast Surgery and Body Contouring August 1993; Santa Fe, New Mexico.
- Silverstein MJ, Mai T, Savalia N, et al. Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol 2014;110:82-9. [Crossref] [PubMed]
- Clinical Practice Guidelines in Oncology. Breast Cancer (NCCN Guidelines) Version 1. 2022 (accessed on October 2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Nanda A, Hu J, Hodgkinson S, et al. Oncoplastic breast-conserving surgery for women with primary breast cancer. Cochrane Database Syst Rev 2021;10:CD013658. [Crossref] [PubMed]
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [Crossref] [PubMed]
- Clough KB, van la Parra RFD, Thygesen HH, et al. Long-term Results After Oncoplastic Surgery for Breast Cancer: A 10-year Follow-up. Ann Surg 2018;268:165-71. [Crossref] [PubMed]
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: A matched-cohort analysis. Eur J Surg Oncol 2016;42:71-7. [Crossref] [PubMed]
- Fitoussi AD, Berry MG, Famà F, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases Plast Reconstr Surg 2010;125:454-62. [outcomes article].
- Banys-Paluchowski M, Kühn T, Masannat Y, et al. Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers (Basel) 2023;15:1173. [Crossref] [PubMed]
- Clough KB, Gouveia PF, Benyahi D, et al. Positive Margins After Oncoplastic Surgery for Breast Cancer. Ann Surg Oncol 2015;22:4247-53. [Crossref] [PubMed]
- Mukhtar RA, Wong J, Piper M, et al. Breast Conservation and Negative Margins in Invasive Lobular Carcinoma: The Impact of Oncoplastic Surgery and Shave Margins in 358 Patients. Ann Surg Oncol 2018;25:3165-70. [Crossref] [PubMed]
- van la Parra RFD, Clough KB, Lejalle-Alaeddine C, et al. Oncoplastic Level 2 Mammoplasty for Large DCIS: 5-Year Results. Ann Surg Oncol 2019;26:2459-65. [Crossref] [PubMed]
- Savioli F, Seth S, Morrow E, et al. Extreme Oncoplasty: Breast Conservation in Patients with Large, Multifocal, and Multicentric Breast Cancer. Breast Cancer (Dove Med Press) 2021;13:353-9. [Crossref] [PubMed]
- Esbona K, Li Z, Wilke LG. Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann Surg Oncol 2012;19:3236-45. [Crossref] [PubMed]
- Volders JH, Haloua MH, Krekel NM, et al. Intraoperative ultrasound guidance in breast-conserving surgery shows superiority in oncological outcome, long-term cosmetic and patient-reported outcomes: Final outcomes of a randomized controlled trial (COBALT). Eur J Surg Oncol 2017;43:649-57. [Crossref] [PubMed]
- Funk A, Heil J, Harcos A, et al. Efficacy of intraoperative specimen radiography as margin assessment tool in breast conserving surgery. Breast Cancer Res Treat 2020;179:425-33. [Crossref] [PubMed]
- Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma in Situ. Pract Radiat Oncol 2016;6:287-95. [Crossref] [PubMed]
- Fitzal F, Bolliger M, Dunkler D, et al. Retrospective, Multicenter Analysis Comparing Conventional with Oncoplastic Breast Conserving Surgery: Oncological and Surgical Outcomes in Women with High-Risk Breast Cancer from the OPBC-01/iTOP2 Study. Ann Surg Oncol 2022;29:1061-70. [Crossref] [PubMed]
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann Surg Oncol 2016;23:3247-58. [Crossref] [PubMed]
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol 2012;38:395-8. [Crossref] [PubMed]
- Mazouni C, Naveau A, Kane A, et al. The role of oncoplastic breast surgery in the management of breast cancer treated with primary chemotherapy. Breast 2013;22:1189-93. [Crossref] [PubMed]
- Clough KB, Lewis JS, Couturaud B, et al. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003;237:26-34. [Crossref] [PubMed]
- McCulley SJ, Macmillan RD. Therapeutic mammaplasty--analysis of 50 consecutive cases. Br J Plast Surg 2005;58:902-7. [Crossref] [PubMed]
- Giacalone PL, Roger P, Dubon O, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol 2007;14:605-14. [Crossref] [PubMed]
- Acree P, Kapadia A, Mahatme R, et al. Review of Current Accepted Practices in Identification of the Breast Lumpectomy Tumor Bed. Adv Radiat Oncol 2022;7:100848. [Crossref] [PubMed]
- Silverstein MJ, Savalia N, Khan S, et al. Extreme oncoplasty: breast conservation for patients who need mastectomy. Breast J 2015;21:52-9. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- van la Parra RFD, Clough KB, Thygesen HH, et al. Oncological Safety of Oncoplastic Level II Mammoplasties After Neoadjuvant Chemotherapy for Large Breast Cancers: A Matched-Cohort Analysis. Ann Surg Oncol 2021;28:5920-8. [Crossref] [PubMed]
- Kaviani A, Safavi A, Mirsharifi R. Immediate and delayed contralateral symmetrization in oncoplastic breast reduction: patients' choices and technique formulation. Plast Reconstr Surg Glob Open 2015;3:e286. [Crossref] [PubMed]
- Deigni OA, Baumann DP, Adamson KA, et al. Immediate Contralateral Mastopexy/Breast Reduction for Symmetry Can Be Performed Safely in Oncoplastic Breast-Conserving Surgery. Plast Reconstr Surg 2020;145:1134-42. [Crossref] [PubMed]
- Grant Y, Thiruchelvam PTR, Kovacevic L, et al. Patient-level costs of staged unilateral versus immediate bilateral symmetrization mammoplasty in breast-conserving surgery. BJS Open 2022;6:zrac073. [Crossref] [PubMed]
- Gabriel SE, Woods JE, O'Fallon WM, et al. Complications leading to surgery after breast implantation. N Engl J Med 1997;336:677-82. [Crossref] [PubMed]
- Koppiker CB, Noor AU, Dixit S, et al. Extreme Oncoplastic Surgery for Multifocal/Multicentric and Locally Advanced Breast Cancer. Int J Breast Cancer 2019;2019:4262589. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Semprini G, Cattin F, Vaienti L, et al. Oncoplastic surgery and cancer relapses: cosmetic and oncological results in 489 patients. Breast 2013;22:946-51. [Crossref] [PubMed]
- Zehnpfennig L, Ritter M, Montagna G, et al. The impact of delayed wound healing on patient-reported outcomes after breast cancer surgery. J Plast Reconstr Aesthet Surg 2022;75:4125-32. [Crossref] [PubMed]
- Nizet JL, Maweja S, Lakosi F, et al. Oncological and surgical outcome after oncoplastic breast surgery. Acta Chir Belg 2015;115:33-41.
- Nakada H, Inoue M, Furuya K, et al. Fat necrosis after breast-conserving oncoplastic surgery. Breast Cancer 2019;26:125-30. [Crossref] [PubMed]
- Al-Hilli Z, Wilkerson A. Breast Surgery: Management of Postoperative Complications Following Operations for Breast Cancer. Surg Clin North Am 2021;101:845-63. [Crossref] [PubMed]
- Ferrando PM, Ala A, Bussone R, et al. Closed Incision Negative Pressure Therapy in Oncological Breast Surgery: Comparison with Standard Care Dressings. Plast Reconstr Surg Glob Open 2018;6:e1732. [Crossref] [PubMed]
- Maggi N, Rais D, Nussbaumer R, et al. The American Society of Breast Surgeons classification system for oncoplastic breast conserving surgery independently predicts the risk of delayed wound healing. Eur J Surg Oncol 2023;49:107032. [Crossref] [PubMed]
- Acea-Nebril B, García-Novoa A, Cereijo-Garea C. Cosmetic sequelae after oncoplastic breast surgery: long-term results of a prospective study. Breast J 2021;27:35-43. [Crossref] [PubMed]
- Tevis SE, James TA, Kuerer HM, et al. Patient-Reported Outcomes for Breast Cancer. Ann Surg Oncol 2018;25:2839-45. [Crossref] [PubMed]
- Liu LQ, Branford OA, Mehigan S. BREAST-Q Measurement of the Patient Perspective in Oncoplastic Breast Surgery: A Systematic Review. Plast Reconstr Surg Glob Open 2018;6:e1904. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Bjelic-Radisic V, Cardoso F, Cameron D, et al. An international update of the EORTC questionnaire for assessing quality of life in breast cancer patients: EORTC QLQ-BR45. Ann Oncol 2020;31:283-8. [Crossref] [PubMed]
- Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997;15:974-86. [Crossref] [PubMed]
- Mendoza TR, Zhao F, Cleeland CS, et al. The validity and utility of the M. D. Anderson Symptom Inventory in patients with breast cancer: evidence from the symptom outcomes and practice patterns data from the eastern cooperative oncology group. Clin Breast Cancer 2013;13:325-34. [Crossref] [PubMed]
- Catsman CJLM, Beek MA, Voogd AC, et al. The COSMAM TRIAL a prospective cohort study of quality of life and cosmetic outcome in patients undergoing breast conserving surgery. BMC Cancer 2018;18:456. [Crossref] [PubMed]
- Aristokleous I, Saddiq M. Quality of life after oncoplastic breast-conserving surgery: a systematic review. ANZ J Surg 2019;89:639-46. [Crossref] [PubMed]
- Pezner RD, Patterson MP, Hill LR, et al. Breast retraction assessment: an objective evaluation of cosmetic results of patients treated conservatively for breast cancer. Int J Radiat Oncol Biol Phys 1985;11:575-8. [Crossref] [PubMed]
- Cardoso MJ, Cardoso J, Amaral N, et al. Turning subjective into objective: the BCCT.core software for evaluation of cosmetic results in breast cancer conservative treatment. Breast 2007;16:456-61. [Crossref] [PubMed]
- O'Connell RL, Stevens RJ, Harris PA, et al. Review of three-dimensional (3D) surface imaging for oncoplastic, reconstructive and aesthetic breast surgery. Breast 2015;24:331-42. [Crossref] [PubMed]
- O'Connell RL, Khabra K, Bamber JC, et al. Validation of the Vectra XT three-dimensional imaging system for measuring breast volume and symmetry following oncological reconstruction. Breast Cancer Res Treat 2018;171:391-8. [Crossref] [PubMed]
- Hadjittofi C, Almalki H, Mirshekar-Syahkal B, et al. Simple oncoplastic breast defect closure improves long-term cosmetic outcome of breast conserving surgery for breast cancer: A randomised controlled trial. Breast 2022;65:104-9. [Crossref] [PubMed]
- Gardfjell A, Dahlbäck C, Åhsberg K. Patient satisfaction after unilateral oncoplastic volume displacement surgery for breast cancer, evaluated with the BREAST-Q™. World J Surg Oncol 2019;17:96. [Crossref] [PubMed]
- Blok YL, Verduijn PS, Corion LUM, et al. An analysis of complication rates and the influence on patient satisfaction and cosmetic outcomes following oncoplastic breast surgery. J Plast Reconstr Aesthet Surg 2022;75:4152-9. [Crossref] [PubMed]
- de Oliveira-Junior I, da Silva IA, da Silva FCB, et al. Oncoplastic Surgery in Breast-Conserving Treatment: Patient Profile and Impact on Quality of Life. Breast Care (Basel) 2021;16:243-53. [Crossref] [PubMed]
- Char S, Bloom JA, Erlichman Z, Jonczyk MM, et al. A comprehensive literature review of patient-reported outcome measures (PROMs) among common breast reconstruction options: What types of breast reconstruction score well? Breast J 2021;27:322-9. [Crossref] [PubMed]
- World Health Organization. Breast cancer. 2021. (Accessed: 6 December 2021). Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- Kedde H, van de Wiel HB, Weijmar Schultz WC, et al. Sexual dysfunction in young women with breast cancer. Support Care Cancer 2013;21:271-80. [Crossref] [PubMed]
- Cobo-Cuenca AI, Martín-Espinosa NM, Sampietro-Crespo A, et al. Sexual dysfunction in Spanish women with breast cancer. PLoS One 2018;13:e0203151. [Crossref] [PubMed]
- Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med 2011;8:294-302. [Crossref] [PubMed]
- Ljungman L, Ahlgren J, Petersson LM, et al. Sexual dysfunction and reproductive concerns in young women with breast cancer: Type, prevalence, and predictors of problems. Psychooncology 2018;27:2770-7. [Crossref] [PubMed]
- Raggio GA, Butryn ML, Arigo D, et al. Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol Health 2014;29:632-50. [Crossref] [PubMed]
- Hanson SE, Lei X, Roubaud MS, et al. Long-term Quality of Life in Patients With Breast Cancer After Breast Conservation vs Mastectomy and Reconstruction. JAMA Surg 2022;157:e220631. [Crossref] [PubMed]
- Char S, Bloom JA, Erlichman Z, et al. How Does Oncoplastic Surgery Compare with Standard Partial Mastectomy? A Systematic Review of Patient-Reported Outcomes. Plast Reconstr Surg 2022;150:950e-8e. [Crossref] [PubMed]
- Mason EJ, Di Leone A, Franco A, et al. Oncoplastic Breast Surgery versus Conservative Mastectomy in the Management of Large Ductal Carcinoma In Situ (DCIS): Surgical, Oncological, and Patient-Reported Outcomes. Cancers (Basel) 2022;14:5624. [Crossref] [PubMed]
- World Health Organization. Promoting Mental Health: Concepts, Emerging Evidence, Practice: A Report of the World Health Organization, Department of Mental Health and Substance Abuse in Collaboration with the Victorian Health Promotion Foundation and the University of Melbourne. World Health Organization; Geneva, Switzerland; 2005.
- Dahlbäck C, Manjer J, Rehn M, et al. Patients Undergoing Breast-Conserving Surgery Can Benefit from the Opportunity to Participate in Choosing Their Surgical Technique. World J Surg 2017;41:734-41. [Crossref] [PubMed]
- Frierson GM, Thiel DL, Andersen BL. Body change stress for women with breast cancer: the Breast-Impact of Treatment Scale. Ann Behav Med 2006;32:77-81. [Crossref] [PubMed]
- Ermoshchenkova MV, Zikiryahodjaev AD, Reshetov IV, et al. Psychological and Aesthetic Outcomes in Breast Cancer Patients. Plast Reconstr Surg Glob Open 2021;9:e3679. [Crossref] [PubMed]
- Tahmasebi S, Mohammadipour M, Ghoddusi Johari M, et al. Determination of Oncologic Outcomes, Satisfaction, and Psychosocial Well-being in Patients with Breast Cancer after Oncoplastic and Conventional Breast Conserving Surgery. World J Plast Surg 2022;11:72-7. [Crossref] [PubMed]
- Chatterjee A, Gass J, Patel K, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3436-44. [Crossref] [PubMed]
- Rendell V, Schmocker R, Abbott DE. Expanding the Scope of Evidence-Based Cancer Care. Surg Oncol Clin N Am 2018;27:727-43. [Crossref] [PubMed]
- Weber WP, Soysal SD, El-Tamer M, et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res Treat 2017;165:139-49. [Crossref] [PubMed]
- Cardoso MJ, Cardoso JS, Vrieling C, et al. Recommendations for the aesthetic evaluation of breast cancer conservative treatment. Breast Cancer Res Treat 2012;135:629-37. [Crossref] [PubMed]
- Rocco N, Catanuto G, Cinquini M, et al. Should oncoplastic breast conserving surgery be used for the treatment of early stage breast cancer? Using the GRADE approach for development of clinical recommendations. Breast 2021;57:25-35. [Crossref] [PubMed]
- Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J 2007;83:777-9. [Crossref] [PubMed]
- Urban C, Anselmi KF, Kroda F, et al. Oncoplasty as the standard of care in breast cancer surgery. Eur Oncol Haematol 2014;10:43-7.
- Esgueva A, Rodríguez-Revuelto R, Espinosa-Bravo M, et al. Learning curves in intraoperative ultrasound guided surgery in breast cancer based on complete breast cancer excision and no need for second surgeries. Eur J Surg Oncol 2019;45:578-83. [Crossref] [PubMed]
- Krekel NM, Lopes Cardozo AM, Muller S, et al. Optimising surgical accuracy in palpable breast cancer with intra-operative breast ultrasound--feasibility and surgeons' learning curve. Eur J Surg Oncol 2011;37:1044-50. [Crossref] [PubMed]
- Tapking C, Kowalewski KF, Hundeshagen G, et al. A Systematic Review of Learning Curves in Plastic and Reconstructive Surgery Procedures. Ann Plast Surg 2020;85:324-31. [Crossref] [PubMed]
- Hung CS, Chang SW, Liao LM, et al. The learning curve of endoscopic total mastectomy in Taiwan: A multi-center study. PLoS One 2017;12:e0178251. [Crossref] [PubMed]
- Carty MJ, Chan R, Huckman R, et al. A detailed analysis of the reduction mammaplasty learning curve: a statistical process model for approaching surgical performance improvement. Plast Reconstr Surg 2009;124:706-14. [Crossref] [PubMed]
- Maruthappu M, Duclos A, Lipsitz SR, et al. Surgical learning curves and operative efficiency: a cross-specialty observational study. BMJ Open 2015;5:e006679. [Crossref] [PubMed]
- Kovacs T, Rubio IT, Markopoulos C, et al. Theoretical and practical knowledge curriculum for European Breast Surgeons. Eur J Surg Oncol 2020;46:717-36. [Crossref] [PubMed]


