Thyroid Langerhans cell histiocytosis and papillary thyroid carcinoma
Introduction
Langerhans cell histiocytosis (LCH) is a clinically variable proliferative disease characterized by accumulation in organs of cells which share phenotypic characteristics with Langerhans’ cells (1,2). LCH may be focal, involve one organ system, or be systemic, affecting multiple organ-systems. Children are the most commonly affected age group, however young adults and especially smokers are at risk of LCH (1-3). Involvement of the pituitary gland is described in about 25% of cases, however concurrent involvement with the thyroid gland is rare (1). We report a rare case of multisystem LCH involving the pituitary and thyroid glands with concurrent papillary thyroid carcinoma metastatic to draining cervical lymph nodes which are also affected by LCH.
Case presentation
A 27-year-old female, married with two children presented to our clinic with a 1-year history of a neck swelling and pressure symptoms on lying backward. The patient had been known as a case of panhypopituitarism for the past 5 years. She initially presented with diabetes insipidus, followed by hyperprolactinemia, secondary amenorrhoea, hypothyroidism and hypogonadotrophic hypogonadism due to a pituitary mass centered in the hypothalamic, sellar and suprasellar regions. She was treated with hormonal replacement therapy. Her clinical course was complicated by an attack of right facial palsy (Ramsay hunts syndrome) which resolved with treatment. She also tested positive for anti-CMV IgM and demonstrated allergies to sulfa-drugs.
Clinical examination revealed a firm, painless, diffusely enlarged thyroid gland, and mobile on swallowing. Laryngoscopy did not identify any structural or functional abnormality of the vocal folds
Blood tests revealed RBC 3.8, Hgb 9.1 gm/dL, Hct 28.4%, Plt 83 and a normal creatinine level. Thyroid function tests revealed secondary hypothyroidism: thyroid stimulating hormone (TSH) level 0.01 mIU/L, free T4 of 13.7 pmol/L, and free T3 of 3.1 pmol/L. She was placed on thyroid hormone replacement therapy. Additional laboratory tests revealed: AST 62 U/L, ALT 29 U/L.
An ultrasound of the abdomen showed hepato-splenomegaly.
An ultrasound of the neck reported bilateral heterogeneous enlarged thyroid lobes and bilateral abnormal suspicious appearance of the cervical and supraclavicular lymph nodes sized between 1.8 to 3.5 cm rounded and hypoechoic.
FNA cytology from the dominant hypoechoic lesion with irregular margins 20 mm in diameter localized in the lower portion of the right thyroid lobe showed papillary thyroid carcinoma in a background of lymphocytic thyroiditis. The patient underwent total thyroidectomy and neck dissection in the form of bilateral central (level VI) + bilateral radical modified lateral neck dissection (levels II–V).
Gross examination of the thyroid gland revealed abnormal dark color and diffuse enlargement due to an infiltrative process with areas of necrosis and hemorrhage occupying both lobes, measuring up to 7 cm in the greatest dimension in each lobe and an enlarged isthmus.
Microscopic examination demonstrated a thyroid with diffuse effacement and infiltration by sheets of epithelioid histiocytic cells with prominent associated lymphoid and eosinophilic component. These cells were identical in morphology to those involving and effacing cervical lymph nodes. The thyroid also demonstrated multiple foci of atypical follicular cells arranged in papillary clusters with prominent nuclear enlargement, overlapping, intranuclear inclusions and grooves. The epithelioid histiocytic component largely obscured the papillary follicular structures which were identifiable as small clusters scattered throughout both lobes of the thyroid.
Immunohistochemical studies demonstrated that the epithelioid histiocytic cells were positive for CD1a, S100, CD68, and CD45. Immunohistochemical stains also helped identify small CKAE1/AE3 positive, TTF1 positive metastatic papillary follicular clusters within pericortical regions of cervical lymph nodes (Figure 1).
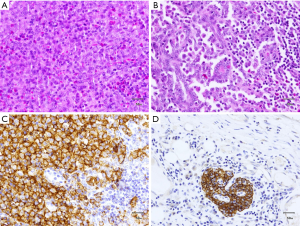
Tumor size was 4 cm in 7 cm lobe and final pathological staging is pT4aN1bMx according to AJCC.
Patient received radioiodine [1-131] adjuvant therapy post-operative.
Following discussion of the case at a multidisciplinary team meeting, the decision was made for continuous treatment with prednisone and chemotherapy.
Discussion
As LCH in the thyroid gland is already considered rare, there are only very few cases reported in the English literature of LCH in the thyroid gland associated with papillary thyroid carcinoma (4-6) (summarized in Table 1).

Full table
LCH may present as a tumour, skin rash, lytic bone lesions, pneumothorax, interstitial lung disease, diabetes insipidus (DI), or present as multiple affected organ systems within the human body as shown in our case in the pituitary and thyroid glands (10).
The etiology of LCH remains unknown. Uncertainty persists as to whether this disorder is primarily neoplastic, immunodysregulatory, or reactive with neoplastic and immunodysregulatory characteristics (6,11).
The increased incidence of papillary carcinoma of the thyroid is well established in inflammatory conditions such as Hashimotos/autoimmune lymphocytic thyroiditis and possibly in Grave’s disease (12,13).
The concurrent findings of LCH and papillary carcinoma in the patient presented here adds to the growing body of literature that suggests that LCH as a neoplastic condition with a prominent inflammatory component may also increase risk of papillary carcinoma in the thyroid adding to the paradigm of inflammation induced neoplasia.
It should also be noted that many papillary carcinomas of the thyroid seem to induce a heavy lymphocytic infiltrate, and on occasion a prominent infiltrate of Langerhan’s type histiocytes, both within the thyroidal parenchyma as well as in draining lymph nodes. It will be interesting to postulate that this induced inflammatory infiltrate may further develop into a neoplastic transformation into LCH (14,15).
We believe that our patient carrying worse prognosis because of co-morbidity than similar patient with the same disease and stage, however no available data comparing the long term prognosis because of the disease rarity.
In conclusion, the clinical varieties of LCH range from a curable, solitary, destructive lesion of bone to a lethal leukemia-like disorder, depending upon the sites and extent of involvement, which primarily affects infants (16). The mainstay of diagnosis of this rare condition is through careful clinic-radiologic and histopathologic analysis supplemented by immunohistochemical staining (S-100, CD1a) (17). Finally, when LCH involves the thyroid gland, careful investigation for co-existent papillary carcinoma should also be performed, as is the case for inflammatory conditions of the thyroid such as Hashimotos thyroiditis and Graves disease.
Acknowledgements
We express our deepest and sincere gratitude to Prof. Marcin Barczynski, Associate Professor of Surgery, at Jagiellonian University, Medical College for his manuscript review and our thanks to Dr. Algarni the consultant surgeon of the case and to Professor Mohamed Satti for his main contribution and guidance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Donadieu J, Chalard F, Jeziorski E. Medical management of langerhans cell histiocytosis from diagnosis to treatment. Expert Opin Pharmacother 2012;13:1309-22. [Crossref] [PubMed]
- Ng-Cheng-Hin B, O'Hanlon-Brown C, Alifrangis C, et al. Langerhans cell histiocytosis: old disease new treatment. QJM 2011;104:89-96. [Crossref] [PubMed]
- Suri HS, Yi ES, Nowakowski GS, et al. Pulmonary langerhans cell histiocytosis. Orphanet J Rare Dis 2012;7:16. [Crossref] [PubMed]
- Coode PE, Shaikh MU. Histiocytosis X of the thyroid masquerading as thyroid carcinoma. Hum Pathol 1988;19:239-41. [Crossref] [PubMed]
- Diamond FB Jr, Shulman DI, Lacson A, et al. Atypical dendritic cell-related histiocytosis with goiter and primary hypothyroidism. J Pediatr 1998;132:357-60. [Crossref] [PubMed]
- Chung DH, Ha SY, Cho HY, et al. Langerhans Cell Histiocytosis in the Thyroid and Draining Lymph Nodes: A Case Report. Endocrinol Metab 2012;27:138-41. [Crossref]
- Schofield JB, Alsanjari NA, Davis J, et al. Eosinophilic granuloma of lymph nodes associated with metastatic papillary carcinoma of the thyroid. Histopathology 1992;20:181-3. [Crossref] [PubMed]
- Safali M, McCutcheon JM, Wright DH. Langerhans cell histiocytosis of lymph nodes: draining a papillary carcinoma of the thyroid. Histopathology 1997;30:599-603. [Crossref] [PubMed]
- Lindley R, Hoile R, Schofield J, et al. Langerhans cell histiocytosis associated with papillary carcinoma of the thyroid. Histopathology 1998;32:180. [Crossref] [PubMed]
- Behrens RJ, Levi AW, Westra WH, et al. Langerhans cell histiocytosis of the thyroid: a report of two cases and review of the literature. Thyroid 2001;11:697-705. [Crossref] [PubMed]
- Abla O, Egeler RM, Weitzman S. Langerhans cell histiocytosis: Current concepts and treatments. Cancer Treat Rev 2010;36:354-9. [Crossref] [PubMed]
- Farbota LM, Calandra DB, Lawrence AM, et al. Thyroid carcinoma in Graves' disease. Surgery 1985;98:1148-53. [PubMed]
- Ott RA, Calandra DB, McCall A, et al. The incidence of thyroid carcinoma in patients with Hashimoto's thyroiditis and solitary cold nodules. Surgery 1985;98:1202-6. [PubMed]
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol 2010;184:4557-67. [Crossref] [PubMed]
- Schröder S, Schwarz W, Rehpenning W, et al. Dendritic/Langerhans cells and prognosis in patients with papillary thyroid carcinomas. Immunocytochemical study of 106 thyroid neoplasms correlated to follow-up data. Am J Clin Pathol 1988;89:295-300. [Crossref] [PubMed]
- Willman CL, Busque L, Griffith BB, et al. Langerhans'-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N Engl J Med 1994;331:154-60. [Crossref] [PubMed]
- Patten DK, Wani Z, Tolley N. Solitary langerhans histiocytosis of the thyroid gland: a case report and literature review. Head Neck Pathol 2012;6:279-89. [Crossref] [PubMed]


