
Preoperative identification of small parathyroid adenomas—better done by fluorocholine positron emission tomography/computed tomography
Highlight box
Key findings
• Small parathyroid adenomas (PAs) are more difficult to locate with conventional imaging than common adenomas. However, choline positron emission tomography/computed tomography (PET/CT) was independent of adenoma size in predicting the correct localization.
What is known and what is new?
• Accurate preoperative localization of PAs is important for successful removal of all diseased tissue and to prevent persistence of the disease. Small adenomas are both more difficult to find intraoperatively and more difficult to localize on preoperative imaging. Cross-sectional imaging methods, including single-photon emission computed tomography/CT and PET/CT, have shown high sensitivity in detecting adenomas.
• For the challenging subset of small adenomas, we discovered a highly significant correlation between an accurate localization and the use of choline PET/CT.
What is the implication, and what should change now?
• Choline PET/CT could serve as a second line imaging modality to prevent missed PAs and subsequent persistence of hyperparathyroidism, especially when they are small.
Introduction
Primary hyperparathyroidism (pHPT) is a common endocrine disorder mainly caused by a solitary parathyroid adenoma (PA). Multiglandular disease (MGD) or hereditary syndromes are less frequent (1). Definitive cure can only be achieved by the surgical removal of the diseased glands with subsequent biochemical cure. Open minimally invasive parathyroidectomy (OMIP) has become the standard procedure to minimize perioperative risks and increase patients’ satisfaction (2). Identification of the PAs is crucial in parathyroid surgery, especially in cases of small PAs or ectopic localization, which occur in up to 16% of cases (3). Dream et al. demonstrated a median maximum gland diameter of 17 mm in 517 resected PAs (4), but smaller glands seem to be increasingly common (5).
Near-infrared light-based intraoperative adjuncts can serve as a real-time verification tool, but they require a visual exposure of the PA being targeted (6). Frozen sections enable identification but carry the risk of damaging healthy glands and extending the operating time. Thus, accurate preoperative imaging diagnostics are crucial to localize PAs and avoid bilateral open neck exploration (BNE).
Preoperative neck ultrasound followed by [99mTc]-sestamibi scintigraphy is a common diagnostic routine with high sensitivity in detecting PAs. When used in conjunction with single-photon computed tomography (SPECT), the diagnostic value of [99mTc]-sestamibi scintigraphy can be further improved, with detection rates up to 88% (7). Sandqvist et al. reported a higher detection rate for SPECT/CT compared to SPECT, especially in small adenomas (<210 mg) (8). However, this advantage is offset by a high radiation dose and the need for an iodine-based agent.
Positron emission tomography/computed tomography (PET/CT) with 18F-fluorocholine analogues promises even higher detection rates, up to >90%, due to its higher resolution (9). These detection rates seem independent of PA size and weight (10). In particular, López-Mora et al. reported superiority in the detecting of small PAs (<10 mm) compared to SPECT/CT and neck ultrasound (11). However, this technology is limited by higher cost and local availability.
To emphasize the importance of accurate preoperative imaging, we present our experience in the preoperative localization of small PAs (≤10 mm) based on a retrospective analysis of 147 consecutive patients undergoing surgery for pHPT. Furthermore, we discuss the potential sequence of preoperative localization diagnostics. We present this article in accordance with the TREND reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-317/rc).
Methods
Patients
Patients undergoing parathyroid resection for pHPT at the Department of General and Visceral Surgery, Cantonal Hospital of Aarau (Aarau, Switzerland), between January 2016 and November 2022 were enrolled in this trial. Data were prospectively added to our own database and, starting from 2018, to the Eurocrine database. The analysis of this data was retrospective. Exclusion criteria were known secondary HPT or congenital syndromes.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The approval of the local ethics committee was obtained (ethics committee of northwestern and central Switzerland; BASEC-ID 2023-00118). General consent or consent for participation in the Eurocrine registry was obtained from all included patients.
Preoperative localization diagnostics
The primary imaging technique, preoperative neck ultrasound, was conducted by the attending endocrinologist, while the secondary imaging technique, SPECT/CT, was performed by the Department of Nuclear Medicine at Cantonal Hospital of Aarau. SPECT/CT images were acquired after the injection of 550 MBq Tc-99m Sestamibi at 20 and 120–150 minutes after injection. In addition, 18F-choline PET/CT was carried out by the in-house Department of Nuclear Medicine. PET/CT images were acquired 20 minutes after the injection of 120–150 MBq 18F-fluorocholine. The costs of all scans were reimbursed by the health insurance companies. For all imaging modalities, the correct quadrant was defined as the correct side plus the correct upper or lower position, whereas the correct lateralization of the diseased parathyroid gland was defined as the prediction of the correct side of the adenoma alone.
Parathyroid surgery
All operations were performed at the Department of General and Visceral Surgery at Cantonal Hospital of Aarau, Switzerland, using Kocher’s incision. Intraoperative PTH (ioPTH) measurements were conducted 10 minutes before and 10 minutes after the removal of the targeted adenoma. A 70% decrease in PTH levels was considered a successful removal of all adenomatous PAs.
Outcome
PTH and albumin-corrected calcium levels were measured on postoperative day (POD) one. The tissue specimens were examined, and the diameter was measured by the local pathologist. Based on the size of the excised PA, patients were divided into two groups: adenomas that were less than or equal to the first quartile (10 mm) were defined as “small”, while those larger than 10 mm were defined as “large”.
Follow-up
Clinical and biochemical follow-up was conducted by the attending endocrinologist. Biochemical follow-up consisted of PTH and albumin-corrected calcium measurements. Cure was defined as normocalcemia (2.15–2.60 mmol/L) for at least six months postoperative. Persistence was defined as elevated PTH (>15.0 ng/L) and calcium (>2.60 mmol/L) levels for at least six months postoperative. Recurrence was defined as recurrent elevated PTH and calcium levels after initial cure.
Statistical analysis
Assuming a normal distribution, P values were determined using a t-test or a Mann-Whitney U test. The chi-squared test was used to examine differences between categorical variables. If there were fewer than five occurrences per category, Fisher’s Exact Test was used. The strength of the correlation between two characteristics was determined using Pearson’s correlation coefficient. The level of significance was taken to be P<0.05. Microsoft Excel (Version 16.7) and IBM SPSS Statistics (Version 28) were used for data collection and analysis.
Results
Preoperative patient and laboratory characteristics
Of the 161 consecutive patients undergoing parathyroidectomy for pHPT, 147 patients met the inclusion criteria and were enrolled in this study (Figure 1). The median diameter of all excised PAs was 15 mm [interquartile range (IQR): 10–20 mm]. One hundred and nine adenomas were classified as “large” (74%), 38 as “small” (26%). There was no difference between the groups in terms of age and sex. Likewise, there was no difference in the preoperative calcium levels and parathyroid hormone levels (Table 1).
Table 1
| Characteristics | Large PGs (n=109) | Small PGs (n=38) | P value |
|---|---|---|---|
| Gender, n [%] | |||
| Male | 27 [25] | 9 [24] | 0.89 |
| Female | 82 [75] | 29 [76] | |
| Age (years) | |||
| Mean ± SD | 63±12 | 59±16 | 0.12 |
| Median [IQR] | 65 [56–73] | 64 [55–71] | |
| Surgery | |||
| Unilateral exploration | 103 | 30 | 0.39 |
| Planed bilateral exploration | 5 | 3 | 0.45 |
| Unplanned bilateral exploration | 1 | 5 | <0.01 |
| Additional thyroid resection | 10 | 10 | <0.01 |
| PA diameter (mm) | |||
| Mean ± SD | 19.61±8.57 | 7.65±2.42 | |
| Median [IQR] | 18 [14–21] | 8 [6–10] | |
| Preoperative laboratory results, mean ± SD | |||
| Calcium (mmol/L) | 2.75±0.21 | 2.72±0.17 | 0.47 |
| PTH (ng/L) | 220±225 | 189±147 | 0.43 |
| First postoperative day laboratory results†, mean ± SD | |||
| Calcium (mmol/L) | 2.32±0.17 | 2.34±0.16 | 0.66 |
| PTH (ng/L) | 47±44 | 42±42 | 0.29 |
†, Six patients did not receive PTH testing on POD1. POD, postoperative day; PG, parathyroid gland; SD, standard deviation; IQR, interquartile range; PA, parathyroid adenoma; PTH, parathyroid hormone.
Localization diagnostics
Preoperative ultrasound as the primary imaging modality was performed with equal frequency in both the small and large PA groups. There was also no difference in the correct lateralization between large and small PAs. However, the prediction of the correct quadrant was significantly weaker in small PAs (P=0.03; Tables 2,3).
Table 2
| Imaging modality | Large PGs (n=109) | Small PGs (n=38) | P value |
|---|---|---|---|
| Ultrasound | 107 [98] | 38 [100] | 0.4 |
| SPECT/CT | 101 [93] | 32 [84] | 0.13 |
| Choline PET/CT | 33 [30] | 23 [61] | <0.01 |
Data are presented as n [%]. Frequency of preoperative localization diagnostics based on adenoma size. PG, parathyroid gland; SPECT/CT, single photon emission computed tomography/computed tomography; PET/CT, positron emission tomography/computed tomography.
Table 3
| Imaging modality | Large PGs (n=109) | Small PGs (n=38) | P value |
|---|---|---|---|
| Ultrasound, n [%] | n=107 | n=38 | |
| Correct quadrant | 56 [52] | 12 [32] | 0.03 |
| Correct lateralization | 66 [62] | 18 [47] | 0.12 |
| Negative localization | 41 [38] | 20 [53] | |
| SPECT/CT, n [%] | n=101 | n=32 | |
| Correct quadrant | 62 [61] | 9 [28] | <0.01 |
| Correct lateralization | 73 [72] | 12 [38] | <0.01 |
| Negative localization | 28 [28] | 20 [63] | |
| Choline PET/CT, n [%] | n=33 | n=23 | |
| Correct quadrant | 27 [82] | 19 [83] | >0.99 |
| Correct lateralization | 31 [94] | 23 [100] | 0.51 |
| Negative localization | 2 [6] | 0 [0] |
The correct quadrant was defined as the correct side plus the correct upper or lower position. The correct lateralization of the diseased PG was defined as the prediction of the correct side of the adenoma alone (including correct quadrant localization). A negative localization was defined when no PG was found, or the localization was wrong. PG, parathyroid gland; SPECT/CT, single photon emission computed tomography/computed tomography; PET/CT, positron emission tomography/computed tomography.
SPECT/CT, as the secondary imaging, was also performed with equal frequency in both groups. For both correct quadrant and correct side, there was a significant positive correlation between large PA size and correct prediction (P<0.01; Tables 2,3).
18F-choline PET/CT was performed in 23 out of 38 (61%) small PAs and 33 out of 109 (30%) large PAs (P<0.01). In 41 of a total of 56 PET/CT scans performed across both groups, it served as a tertiary imaging modality (Figure 2), and in seven cases it was the secondary imaging modality (due to radiation protection or patient refusal). Eight scans were conducted prior to surgery for recurrent or persistent HPT. While the detection performance was independent of the adenoma size (Table 3), the subgroup of small adenomas showed a highly significant better localization by choline PET/CT for both the correct side and the correct quadrant (P<0.0001). The subgroup of large adenomas showed slightly worse but still significantly better localization by PET/CT for both the correct side and the correct quadrant (P<0.01; Table 4).
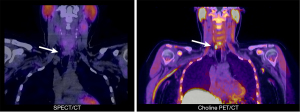
Table 4
| PA size | Ultrasound | SPECT/CT | Choline PET/CT | P value |
|---|---|---|---|---|
| Small PA, n [%] | n=38 | n=32 | n=23 | |
| Correct quadrant | 12 [32] | 9 [28] | 19 [83] | <0.0001 |
| Correct lateralization | 18 [47] | 12 [38] | 23 [100] | <0.0001 |
| Negative localization | 20 [53] | 20 [63] | 0 [0] | |
| Large PA, n [%] | n=107 | n=101 | n=33 | |
| Correct quadrant | 56 [52] | 62 [61] | 27 [82] | <0.01 |
| Correct lateralization | 66 [62] | 73 [72] | 31 [94] | <0.01 |
| Negative localization | 41 [38] | 28 [28] | 2 [6] |
The correct quadrant was defined as the correct side plus the correct upper or lower position. The correct lateralization of the diseased PG was defined as the prediction of the correct side of the adenoma alone (including correct quadrant localization). A negative localization was defined when no PG was found, or the localization was wrong. PA, parathyroid adenoma; SPECT/CT, single photon emission computed tomography/computed tomography; PET/CT, positron emission tomography/computed tomography; PG, parathyroid gland.
Parathyroid surgery
All procedures were performed by six different surgeons. Of the 147 procedures, 131 were performed by European Board of Surgery qualified (EBSQ) endocrine surgeons. Among the patients, 133 OMIP, while planned BNE was conducted in eight cases. Additionally, conversion from OMIP to BNE was necessary in six patients. In 20 patients, parathyroidectomy was combined with thyroid resection for multinodular goiter (11 patients) or presumed intrathyroidal PA (9 patients). There were no cases requiring conversion to sternotomy or revision surgery for postoperative hemorrhage.
MGD
MGD was detected during surgery in 10 out of 147 patients (6.8%), always consisting of two adenomas. Among those ten patients, eight of the 20 PAs found were categorized as “small” (40%), and 12 were categorized as “large” (60%). MGD was correctly presumed by ultrasound in one out of nine patients (11%), by SPECT/CT in three out of 10 patients (30%), and by PET/CT in five out of six patients (83%). Out of the 11 missed PAs by ultrasound, seven were categorized as “small” (Table 5). Equally to singular adenomas, there was a highly significant correlation between a correct detection of the exact location of the adenoma and Choline PET/CT as preoperative imaging (P<0.01; Table 6).
Table 5
| Patient ID | Size in mm | Ultrasound | SPECT/CT | Choline PET/CT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PA1 | PA2 | Correct (n=7) | Negative (n=11) | Correct (n=9) | Negative (n=11) | Correct (n=11) | Negative (n=1) | ||||
| 6 | 11 | 11 | 1 | 1 | 1 | 1 | 2 | 0 | |||
| 82 | 24 | 10 | 1 | 1 | 2 | 0 | NA | NA | |||
| 85 | 30 | 24 | NA | NA | 2 | 0 | NA | NA | |||
| 93 | 10 | 10 | 0 | 2 | 0 | 2 | 1 | 1 | |||
| 104 | 17 | 9 | 1 | 1 | 1 | 1 | 2 | 0 | |||
| 108 | 13 | 12 | 0 | 2 | 0 | 2 | 2 | 0 | |||
| 125 | 8 | 9 | 1 | 1 | 0 | 2 | 2 | 0 | |||
| 128 | 18 | 10 | 1 | 1 | 1 | 1 | NA | NA | |||
| 140 | 13 | 5 | 0 | 2 | 0 | 2 | 2 | 0 | |||
| 156 | 25 | 21 | 2 | 0 | 2 | 0 | NA | NA | |||
Size and identification by imaging modality per patient in patients with multiglandular disease (always containing of two adenomas). Correct localization defined a correctly predicted quadrant. Cells marked “NA”: not performed. ID, patient identification number; SPECT/CT, single photon emission computed tomography/computed tomography; PET/CT, positron emission tomography/computed tomography; PA1, parathyroid adenoma number one; PA2, parathyroid adenoma number two.
Table 6
| Localization | Ultrasound (n=18) | SPECT/CT (n=20) | Choline PET/CT (n=12) | P value |
|---|---|---|---|---|
| Correct | 7 [39] | 9 [45] | 11 [92] | <0.01 |
| Negative | 11 [61] | 11 [55] | 1 [8] |
Data are presented as n [%]. Correct preoperative quadrant prediction of PAs in MGD. Fisher’s exact test was performed to determine the level of significance. SPECT/CT, single photon emission computed tomography/computed tomography; PET/CT, positron emission tomography/computed tomography; PA, parathyroid adenoma; MGD, multiglandular disease.
Follow-up
Median follow-up for all patients was 13 months (IQR: 4–54 months) with a biochemical cure rate of 91%. In patients with large PAs, median follow-up was 16 months (IQR: 4–54 months), respectively 12 months (IQR: 4–43 months) in patients with small PAs (P=0.2). Biochemical cure was achieved in 83% of all followed-up patients with small PAs and in 94% of all followed-up patients with large PAs (P=0.08). Recurrence after six months occurred in one patient with a large PA. Persistent HPT occurred significantly more often in patients with small PAs (17% vs. 5% in large PAs, P=0.04). There was no difference in calcium or PTH levels at the latest follow-up. Five patients with large PA (4.6%), respectively five patients with small PA (13%) were lost to follow-up (Table 7).
Table 7
| Characteristics | Large PAs (n=109) | Small PAs (n=38) | P value |
|---|---|---|---|
| Follow-up, n [%] | |||
| Yes | 93 [85] | 29 [76] | 0.2 |
| No | 16 [15] | 9 [24] | |
| Latest follow-up (months) | |||
| Mean ± SD | 44±62 | 40±67 | 0.72 |
| Median [IQR] | 16 [4–54] | 12 [4–43] | |
| Clinical results, n [%] | |||
| Cure | 87 [94] | 24 [83] | 0.08 |
| Recurrence | 1 [1] | 0 | 0.58 |
| Persistence | 5 [5] | 5 [17] | 0.04 |
| Lab results at latest follow-up | |||
| Calcium (mmol/L) | 2.43 | 2.41 | 0.43 |
| PTH (ng/L) | 79 | 78 | 0.87 |
PA, parathyroid adenoma; SD, standard deviation; IQR, interquartile range; PTH, parathyroid hormone.
Discussion
Several studies describe a decrease in PA size in recent decades (5,12). Therefore, preoperative localization diagnostics are crucial for successful intraoperative identification, especially in small adenomas.
We found no difference in preoperative calcium and PTH levels between the analyzed groups. In contrast, Dream et al. showed a positive correlation between gland size and preoperative calcium and PTH levels (4). Naples et al. found only a poor correlation between gland volume and preoperative calcium and PTH levels in a large series of >2,000 cases (13).
Cervical ultrasound is essential as a basic imaging modality, as it is cost-effective and widely available. Berber et al. demonstrated that the volume of an individual PA is the strongest predictive variable affecting ultrasound detection sensitivity (14). Likewise, the data from this study show a significantly worse quadrant detection rate in small adenomas (32% vs. 52% in in large adenomas, P=0.03). In patients with MGD, which is associated with small PAs (5), the detection rate of the adenomas by ultrasound (correct quadrant) was only 39%, with only one of eight small PAs detected (12.5%). This corresponds to detection rates reported by Ruda et al. and Medas et al., who described sensitivities of 16.2% and 18.7% in patients with double adenomas and MGD, respectively (15,16). In a series of 254 patients with MGD by Shah et al., two or more suspected PAs were identified by ultrasound in 36 cases (14.2%) (17). However, it should be noted, that ultrasound is highly operator-dependent, and comparisons among studies are questionable (18). Thyroid pathologies can also affect the detection rate, but this was not considered in this study (16). For these reasons, the European Association of Nuclear Medicine (EANM) guideline for parathyroid imaging recommends the combination of ultrasound with scintigraphy (19).
The PA-size dependent detection rate is even more evident with SPECT/CT, with a significantly worse detection rate for small adenomas for both the correct side and quadrant (P<0.01). Similarly, Qiu et al. reported a significant correlation between PA size and correct quadrant localization in a series of 244 patients with pHPT but found no advantages of SPECT/CT over planar MIBI scintigraphy (20). If the maximum PA diameter was less than 10 mm, they found positive 99mTc-MIBI scans in 13 out of 72 patients (18%), compared to 161 out of 172 (94%) in patients with a PA diameter >10 mm. Several other studies underline the weakness of this technique in detecting small PAs (8,14,21). Our detection rate in patients with MGD was lower than in single gland disease (45% vs. 60%), supporting the finding that SPECT/CT is less sensitive in patients with MGD (22).
This difficulty in detecting small PAs does not appear to be as pronounced with choline PET/CT. In a prospective study of 33 patients with negative SPECT/CT imaging for pHPT, López-Mora et al. were able to visualize the targeted PA by PET/CT in 30 cases. The median diameter of the PAs missed on SPECT/CT was 13 mm. Interestingly, a digital PET/CT showed a significantly higher detection rate than an analog system. All PAs detected by the digital system alone were <10 mm (11). Several other articles also describe the non-inferiority of the modality in detecting small PAs, as well as its superiority compared to SPECT/CT (10,23,24). In accordance with these results, we found equal detection rates for both the correct side and the correct quadrant for small and large PAs. However, PET/CT was performed significantly more often in patients with small PAs (P<0.01). In five out of six patients with MGD who underwent choline PET/CT, eleven out of twelve PAs were exactly localized (92%).
In line with numerous studies, there is a clear superiority of choline PET/CT over SPECT/CT in detecting the exact localization of a targeted PA (23,24).
Our data support these previous findings: for the subgroup of patients with MGD and even more clearly for the subgroup of patients with small PAs, there was a highly significant correlation between performing choline PET/CT and correct quadrant localization of the targeted PA(s).
Despite the demonstrated superiority of choline PET/CT over SPECT/CT, it has not yet gained wide acceptance as a secondary imaging modality. This is due, among other things, to the up to 3 times higher costs per examination. Additionally, PET scanners are not widely available, and the costs are rarely covered by insurance companies.
However, the clinical short-term and long-term outcomes are the decisive factors for the patient. We observed a 94% cure rate in patients with large PAs and a slightly lower cure rate of 83% in patients with small PAs, which is comparable to published data (25). Persistence occurred significantly more often in small PAs (P=0.04), but the low number of patients (n=5 vs. n=5) weakens the evidence (Table 7: follow-up). Dream et al. also demonstrated a trend towards higher cure rates in larger PAs but with no significant difference (4).
The surgeon’s expertise is most likely the key factor, with a strong correlation between case volume and outcome (26). Although one study demonstrated that parathyroid surgery could be performed successfully even in low-volume clinics with correct preoperative localization diagnostics, the rate of re-operations is significantly lower in high-volume clinics (27,28). However, when high-volume clinics are not available due to structural circumstances, correct preoperative localization seems essential for successful surgery, especially in revision surgery, MGD, or ectopic PAs.
The retrospective study design has several limitations: PET/CT was mainly performed in patients with negative or equivocal SPECT/CT. Therefore, a direct comparison of the imaging modalities is difficult. SPECT/CT and PET/CT images were interpreted by a single radiologist, and ultrasound was performed and interpreted by a single endocrinologist. Parathyroidectomies were performed by six different surgeons, but based on their long experience, we do not suspect any influence on the outcome. As mentioned above, thyroid abnormalities or ectopic positions were not analyzed, which may have an impact on preoperative detection rates.
Conclusions
Preoperative identification of small PAs is overall challenging, but essential for successful surgical resection. Ultrasound is indispensable and widely available as a basic diagnostic tool. However, it is operator dependent and inaccurate in detecting small PAs. Given that choline PET/CT is highly significant for accurate localization compared to other imaging modalities and has a high detection rate for all PAs regardless of size and location, this technique should be considered as a second-line imaging modality. Especially in low-volume clinics, it could help to safely perform HPT surgery and avoid recurrence surgery and its associated complications. However, its high cost and limited availability remain unresolved.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-317/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-317/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-317/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-317/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by regional ethics committee of northwestern and central Switzerland (BASEC-ID 2023-00118). Written informed consent was obtained from all individuals participating in this trial.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet 2018;391:168-78. [Crossref] [PubMed]
- Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg 2016;151:959-68. [Crossref] [PubMed]
- Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg 2006;191:418-23. [Crossref] [PubMed]
- Dream S, Yen TWF, Doffek K, et al. Variation in parathyroid adenoma size in patients with sporadic, primary hyperparathyroidism: small gland size does not preclude single gland disease. Langenbecks Arch Surg 2022;407:2067-73. [Crossref] [PubMed]
- McCoy KL, Chen NH, Armstrong MJ, et al. The small abnormal parathyroid gland is increasingly common and heralds operative complexity. World J Surg 2014;38:1274-81. [Crossref] [PubMed]
- Wolf HW, Grumbeck B, Runkel N. Intraoperative verification of parathyroid glands in primary and secondary hyperparathyroidism using near-infrared autofluorescence (IOPA). Updates Surg 2019;71:579-85. [Crossref] [PubMed]
- Pata G, Casella C, Magri GC, et al. Financial and clinical implications of low-energy CT combined with 99m Technetium-sestamibi SPECT for primary hyperparathyroidism. Ann Surg Oncol 2011;18:2555-63. [Crossref] [PubMed]
- Sandqvist P, Farnebo J, Nilsson IL, et al. The preoperative localisation of small parathyroid adenomas improves when adding Tc-99m-Sestamibi SPECT to multiphase contrast-enhanced CT. Insights Imaging 2021;12:72. [Crossref] [PubMed]
- Treglia G, Piccardo A, Imperiale A, et al. Diagnostic performance of choline PET for detection of hyperfunctioning parathyroid glands in hyperparathyroidism: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2019;46:751-65. [Crossref] [PubMed]
- Grimaldi S, Young J, Kamenicky P, et al. Challenging pre-surgical localization of hyperfunctioning parathyroid glands in primary hyperparathyroidism: the added value of (18)F-Fluorocholine PET/CT. Eur J Nucl Med Mol Imaging 2018;45:1772-80. [Crossref] [PubMed]
- López-Mora DA, Sizova M, Estorch M, et al. Superior performance of 18F-fluorocholine digital PET/CT in the detection of parathyroid adenomas. Eur J Nucl Med Mol Imaging 2020;47:572-8. [Crossref] [PubMed]
- Almquist M, Bergenfelz A, Mårtensson H, et al. Changing biochemical presentation of primary hyperparathyroidism. Langenbecks Arch Surg 2010;395:925-8. [Crossref] [PubMed]
- Naples R, Thomas JD, Monteiro R, et al. Preoperative Calcium and Parathyroid Hormone Values Are Poor Predictors of Gland Volume and Multigland Disease in Primary Hyperparathyroidism: A Review of 2000 Consecutive Patients. Endocr Pract 2022;28:77-82. [Crossref] [PubMed]
- Berber E, Parikh RT, Ballem N, et al. Factors contributing to negative parathyroid localization: an analysis of 1000 patients. Surgery 2008;144:74-9. [Crossref] [PubMed]
- Ruda JM, Hollenbeak CS, Stack BC Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 2005;132:359-72. [Crossref] [PubMed]
- Medas F, Erdas E, Longheu A, et al. Retrospective evaluation of the pre- and postoperative factors influencing the sensitivity of localization studies in primary hyperparathyroidism. Int J Surg 2016;25:82-7. [Crossref] [PubMed]
- Shah US, McCoy KL, Kelley ML, et al. How and when is multiglandular disease diagnosed in sporadic primary hyperparathyroidism? Surgery 2022;171:35-9. [Crossref] [PubMed]
- Tay D, Das JP, Yeh R. Preoperative Localization for Primary Hyperparathyroidism: A Clinical Review. Biomedicines 2021;9:390. [Crossref] [PubMed]
- Petranović Ovčariček P, Giovanella L, Carrió Gasset I, et al. The EANM practice guidelines for parathyroid imaging. Eur J Nucl Med Mol Imaging 2021;48:2801-22. [Crossref] [PubMed]
- Qiu ZL, Wu B, Shen CT, et al. Dual-phase (99m)Tc-MIBI scintigraphy with delayed neck and thorax SPECT/CT and bone scintigraphy in patients with primary hyperparathyroidism: correlation with clinical or pathological variables. Ann Nucl Med 2014;28:725-35. [Crossref] [PubMed]
- Kedarisetty S, Fundakowski C, Ramakrishnan K, et al. Clinical Value of Tc99m-MIBI SPECT/CT Versus 4D-CT or US in Management of Patients With Hyperparathyroidism. Ear Nose Throat J 2019;98:149-57. [Crossref] [PubMed]
- Nichols KJ, Tomas MB, Tronco GG, et al. Sestamibi parathyroid scintigraphy in multigland disease. Nucl Med Commun 2012;33:43-50. [Crossref] [PubMed]
- Beheshti M, Hehenwarter L, Paymani Z, et al. (18)F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with (99m)Tc-MIBI or (99m)Tc-tetrofosmin SPECT/CT: a prospective dual-centre study in 100 patients. Eur J Nucl Med Mol Imaging 2018;45:1762-71. [Crossref] [PubMed]
- Thanseer N, Bhadada SK, Sood A, et al. Comparative Effectiveness of Ultrasonography, 99mTc-Sestamibi, and 18F-Fluorocholine PET/CT in Detecting Parathyroid Adenomas in Patients With Primary Hyperparathyroidism. Clin Nucl Med 2017;42:e491-7. [Crossref] [PubMed]
- Weber T, Dotzenrath C, Dralle H, et al. Management of primary and renal hyperparathyroidism: guidelines from the German Association of Endocrine Surgeons (CAEK). Langenbecks Arch Surg 2021;406:571-85. [Crossref] [PubMed]
- Iacobone M, Scerrino G, Palazzo FF. Parathyroid surgery: an evidence-based volume-outcomes analysis: European Society of Endocrine Surgeons (ESES) positional statement. Langenbecks Arch Surg 2019;404:919-27. [Crossref] [PubMed]
- Chow TL, Choi CY, Lam SH. Focused parathyroidectomy without intra-operative parathyroid hormone monitoring for primary hyperparathyroidism: results in a low-volume hospital. J Laryngol Otol 2015;129:788-94. [Crossref] [PubMed]
- Mitchell J, Milas M, Barbosa G, et al. Avoidable reoperations for thyroid and parathyroid surgery: effect of hospital volume. Surgery 2008;144:899-906; discussion 906-7. [Crossref] [PubMed]



