
Contralateral prophylactic mastectomy for unilateral breast cancer in Chinese female population: a retrospective cohort study
Highlight box
Key findings
• Contralateral prophylactic mastectomy (CPM) does not provide any overall survival and disease-free survival benefits compared to unilateral mastectomy. CPM surgical decision-making is independently associated with lower tumor-node-metastasis staging and nipple-sparing mastectomy surgical protocol. The appearance satisfaction with CPM procedure was relatively low.
What is known and what is new?
• Despite the current increase in the proportion of female unilateral breast cancer patients choosing CPM, there is still a lack of validated evidence that CPM improves long-term survival in average-risk patients.
• Most of the current studies on CPM are based on patients from Europe and the United States, and there are relatively limited data based on Asian populations. This research explored the application trend, survival benefits, decision-making factors, and satisfaction of CPM in Chinese population.
What is the implication, and what should change now?
• Clinicians should provide adequate reference information on CPM, especially about survival and appearance, to guide patients to make more rational surgical decisions.
Introduction
Contralateral prophylactic mastectomy (CPM) is a risk-reducing mastectomy (RRM) by removing the contralateral uninvolved breast for patients with unilateral breast cancer (UBC) to prevent the development of second breast cancer (1,2). Based on data from the Surveillance, Epidemiology, and End Results (SEER) registry, the proportion of patients with UBC undergoing CPM has increased from 3.9% to 12.7% between 2002 and 2012 (3). In an American study evaluating 2015–2020 data from a large administrative claims database, the annual CPM trends in UBC patients increased from 3.4% in 2016 to 6.8% in 2019, and the proportion of younger patients was significantly higher and increased faster than older women (4). CPM is also used in the Asian population but at a much lower rate. A study from Singapore’s largest healthcare organization indicated that the highest rate of CPM from 2001–2010 was only 1.26% (5), while it is unclear for other Asian countries.
To date, several studies have demonstrated that the surgical risk-reduction strategy of CPM is increasingly popular, but its effectiveness in improving the survival benefit for the vast majority of patients with UBC is controversial. It is demonstrated that CPM significantly reduced the risk of CBC and the incidence of distant metastases (DMs) in UBC patients, but there is insufficient evidence suggesting that CPM can improve survival in females at average risk (6-8). A Cochrane systematic review for RRM showed that as of 2018, 26 studies consistently reported a decrease in the incidence of CBC with CPM, but the improvement in OS, and breast cancer-specific survival (BCSS) was inconsistent. The survival benefit of CPM disappeared when multiple studies were matched for analysis, suggesting that the survival benefit may be limited to certain subgroups of patients (7).
Current guidelines from the National Comprehensive Cancer Network (NCCN) and the American Society of Breast Surgeons (ASBrS) state that CPM should be considered for patients at high risk for contralateral breast cancer (CBC), such as those with BRCA1/2 mutations or strong family history (9-12). However, Hawley et al. (13) found that only 31% of all females undergoing CPM had a BRCA1/2 mutation or a strong family history. Instead, the decision-making of CPM is most often motivated by the patient’s fear and anxiety about future breast cancer diagnosis and cosmetic concerns, such as asymmetry after unilateral mastectomy (UM) (14,15). Furthermore, Fayanju et al. (16) retrieved studies on CPM published before March 2012 for meta-analysis and concluded that no significant survival benefit was observed with CPM in patients at high risk with BRCA1/2 mutation carriers or a family history of breast cancer. Due to differences in socioeconomic and cultural backgrounds, as well as cancer diagnosis and treatment, the characteristics and prognosis of Asian female patients choosing CPM are likely to be different from Western patients.
Knowledge gap and objective
To fill the research gap of CPM in Asian populations, this study was conducted to analyze the application trend and decision-making factors of CPM and compare patient characteristics and survival outcomes between CPM and other breast surgery procedures in Chinese UBC patients who underwent breast surgery at our medical institution. Further analyses were conducted to explore the specific patient subgroups benefiting from CPM, post-CPM satisfaction, and impact on quality of life. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-384/rc).
Methods
This retrospective cohort study from a single-institution was approved by the Ethics Committee of The First Medical Center of Chinese PLA General Hospital (No. S2020-451-01), and the requirement for individual consent was waived due to the retrospective nature of the analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Participants
The patients undergoing breast surgery in The First Medical Center of Chinese PLA General Hospital from January 1, 2005 to December 31, 2017 were collected. And the patients enrolled were required to meet all inclusion criteria and were excluded if any of the exclusion criteria were met.
The inclusion criteria were as follows: (I) female; (II) breast lesion on the affected side confirmed by histopathology as in situ or invasive breast cancer preoperatively without DM; (III) no suspicious malignant lesions in the contralateral breast with the Breast Imaging Reporting and Data System (BI-RADS) category 1–3, or BI-RADS category 4 but pathologically confirmed as benign; (IV) surgery for breast cancer performed in our hospital.
The exclusion criteria were as follows: (I) specific types of breast cancer, such as mucinous carcinoma, carcinoma with apocrine differentiation, metaplastic carcinoma, rare and salivary gland-type tumors, and neuroendocrine neoplasms; (II) lobular carcinoma in situ; (III) preoperative contralateral breast imaging indicated the lesion with BI-RADS grade 1–3, but intraoperative excisional biopsy confirmed malignancy; (IV) significant clinicopathological data absence or follow-up records incomplete.
Patients were divided into four groups according to breast surgery procedures: (I) CPM group: the affected breast received therapeutic surgery and the contralateral breast received prophylactic surgery, including simple mastectomy (SM) and nipple-sparing mastectomy (NSM); (II) SM group: the affected breast received therapeutic SM surgery and the contralateral breast had no intervention; (III) NSM group: the affected breast received therapeutic NSM surgery and the contralateral breast had no intervention; (IV) breast-conserving surgery (BCS) group: the affected breast received therapeutic BCS surgery and the contralateral breast had no intervention. Since the surgical procedures of patients in the CPM group only include SM and NSM, in order to ensure comparability between the groups, patients in the SM and NSM groups were combined into a UM group.
Clinicopathological factors and definitions
For each patient, demographic data, including age, body mass index (BMI), menstrual status, family history of malignancy, and contralateral breast events were retrieved.
BMI was calculated as weight in kilograms divided by height in meters squared. A BMI <18.5 kg/m2 is considered underweight, a BMI between ≥18.5 and <24 kg/m2 is normal, a BMI between ≥24 and <28 kg/m2 is overweight, and a BMI ≥28 kg/m2 is obesity (17). The patient’s menopausal status was determined according to NCCN guidelines (18). Patients were considered to have a family history of breast cancer or other malignancies if one or more first- or second-degree relatives had breast cancer or other malignancies. Contralateral breast events are defined as the BI-RADS category of breast lesions in the contralateral breast obtained from imaging reports at the time of initial diagnosis of primary breast cancer.
Breast cancer characteristics included year and age of diagnosis, laterality, tumor size (T stage), nodal status (N stage), tumor-node-metastasis (TNM) stage, tumor grade (G), receptor status, and Ki67 index. The pathological TNM staging of tumors was defined according to the staging of the American Joint Committee on Cancer (AJCC) (19). Tumor grades for invasive breast cancer were classified as low grade (G1), intermediate grade (G2), and high grade (G3) based on the Nottingham grading system. Estrogen receptor (ER) and progesterone receptor (PR) positivity were defined as ≥1% of tumor cells positive on immunohistochemistry (IHC). For human epidermal growth factor receptor 2 (HER2) strength of staining, 1+ or no expression for negative, 3+ expression for positive. The expression of HER2 in 2+ was further evaluated based on fluorescence in situ hybridization (FISH) (20). Ki67 was categorized as low-grade (<15%), medial-grade (≥15–≤30%), or high-grade (>30%) based on positive nuclei.
Follow-up
OS and disease-free survival (DFS) were selected as survival outcome indicators. OS was calculated as the date of surgery to death due to any cause or the last known survival date. DFS was calculated as the date of surgery to tumor recurrence or metastasis (local or distant) or death or last known survival date. All patients were followed through January 29, 2022 and the follow-up staff had no knowledge of the patients’ identifying information or clinicopathological information.
For the assessment of patients satisfaction and the extent to the life quality of CPM, based on the Breast-Q scoring system (21) and combining the practical maneuverability of questionnaires in our hospital, we developed a follow-up scoring scale, mainly including: (I) the identity of the proposer, i.e., the identity of the person who proposed CPM before surgery, divided into doctors and patients themselves; (II) overall satisfaction, i.e., the satisfaction with the overall post-operative situation, and appearance satisfaction, i.e., satisfaction with the current appearance of the breast, which can be divided into very satisfied, basic satisfaction, dissatisfaction and regret to receive CPM; (III) impact on quality of life, including family emotional life, sexual life, work situation, social activities, physical labor, daily exercise, which can be divided into unaffected, mildly affected, moderately affected and severe impact.
For patient survival status, annual follow-up was performed by telephone. The satisfaction and quality of life assessments were performed at 2 years after CPM by outpatient.
Statistical analysis
All statistical analysis was performed using SPSS software (version 21, IBM) and R software (version 3.3.0) and a two-sided P value <0.05 was considered statistically significant.
Continuous variables are expressed as mean and standard deviation and compared using the Wilcoxon rank sum test, and categorical variables are expressed as percentages and compared using the Chi-squared test. The Cochran-Armitage trend test was used for longitudinal comparison of the proportion of different surgical procedures within the study period. The life-table methods were used to calculate 5- and 10-year OS and DFS. The survival curves were plotted with Kaplan-Meier (KM) survival analysis and compared with the log-rank test. Univariate and multivariate Cox regression hazard analyses were performed to investigate clinicopathological factors associated with prognosis and provide hazard ratios (HRs) and 95% confidence intervals (CIs). Univariate and multivariate logistic regression analyses were performed to assess the correlation between various clinicopathological characteristics of patients and patients’ CPM surgical decisions and provide odds ratios (ORs) and 95% CI.
To reduce selection bias and confounding between UM and CPM patients, a propensity score matching (PSM) technique with a 1:1 matching ratio was used to match the two groups. The clinical variables, including age, BMI, menstrual status, family history of malignancy, and pathological factors, including receptor status, Ki67 index, tumor grade, and T, N, and TNM stage, were used in the PSM. Survival analysis was performed again after PSM and subgroup analysis according to patients’ clinicopathological factors was conducted.
Results
Participants characteristics
A total of 6,162 breast cancer patients underwent breast surgery at The First Medical Center of Chinese PLA General Hospital between January 1, 2005, and December 31, 2017. As shown in Figure 1, screening was performed according to the inclusion and exclusion criteria, resulting in 4,276 eligible patients with UBC. Of these, 4,203 patients (98.3%) underwent non-CPM procedures, including 3,567 with SM, 151 with NSM and 485 with BCS, and 73 patients (1.7%) underwent CPM, including 38 patients with SM and 35 patients with NSM.
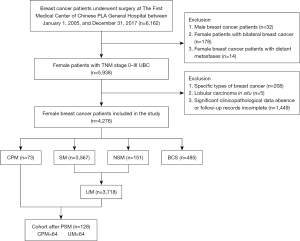
The mean age at first diagnosis of breast cancer for the 4,276 patients was 49.79±10.82 years, with those in the CPM, SM, NSM, and BCS groups being 41.72±11.90, 50.54±10.50, 41.23±6.91, and 48.19±12.05 years, respectively, and the mean age of UM group combined with SM group and NSM group was 50.00±10.64 years. The baseline characteristics of patients were statistically described by the procedure type (Table 1). In the comparison between the CPM and UM groups, the distribution of patients in both groups was balanced at the level of family history of other malignancies (P=0.825) and tumor N stage (P=0.078) only, and the rest of the distribution at all characteristics was significantly different (P<0.05). The CPM group had a greater proportion of younger patients under 45 years of age (60.3% vs. 34.0%), premenopausal patients (58.9% vs. 46.6%), and patients with a family history of breast cancer (17.8% vs. 3.4%) and positive CBC events (28.7% vs. 12.3%). No significant difference was detected in mortality between the two groups.
Table 1
| Patient characteristics | CPM (n=73) | UM (n=3,718) | P value |
|---|---|---|---|
| Age (years) | <0.001 | ||
| <45 | 44 | 1,266 | |
| ≥45–<60 | 23 | 1,770 | |
| ≥60 | 6 | 682 | |
| BMI (kg/m2) | 0.007 | ||
| <18.5 | 5 | 89 | |
| ≥18.5–<24 | 44 | 1,716 | |
| ≥24–<28 | 19 | 1,386 | |
| ≥28 | 5 | 514 | |
| Unknown | 0 | 13 | |
| Menopausal state | 0.043 | ||
| Premenopausal | 43 | 1,734 | |
| Postmenopausal | 7 | 742 | |
| Unknown | 23 | 1,242 | |
| Family history of breast cancer | <0.001 | ||
| Yes | 13 | 125 | |
| No | 60 | 3,593 | |
| Family history of other malignancies | 0.825 | ||
| Yes | 6 | 376 | |
| No | 67 | 3,337 | |
| Unknown | 0 | 5 | |
| Contralateral breast events | 0.001 | ||
| 0 | 25 | 1,766 | |
| 2 | 2 | 39 | |
| 3 | 14 | 335 | |
| 4 | 5 | 85 | |
| Unknown | 27 | 1,493 | |
| ER | <0.001 | ||
| Positive | 43 | 2,342 | |
| Negative | 11 | 1,054 | |
| Unknown | 19 | 322 | |
| PR | <0.001 | ||
| Positive | 51 | 2,352 | |
| Negative | 8 | 1,058 | |
| Unknown | 14 | 308 | |
| HER2 | <0.001 | ||
| Positive | 13 | 720 | |
| Negative | 20 | 1,444 | |
| 2+ | 21 | 1,235 | |
| Unknown | 19 | 319 | |
| Ki67 | <0.001 | ||
| Low | 11 | 460 | |
| Medial | 18 | 1,007 | |
| High | 21 | 1,875 | |
| Unknown | 23 | 376 | |
| Grade | 0.004 | ||
| 1 | 4 | 102 | |
| 2 | 32 | 1,617 | |
| 3 | 7 | 953 | |
| Unknown | 30 | 1,046 | |
| T stage | 0.001 | ||
| 0 | 12 | 215 | |
| 1 | 28 | 1,562 | |
| 2 | 19 | 1,342 | |
| 3/4 | 2 | 169 | |
| Unknown | 12 | 430 | |
| N stage | 0.078 | ||
| 0 | 41 | 1,945 | |
| 1 | 13 | 865 | |
| 2 | 6 | 442 | |
| 3 | 4 | 265 | |
| Unknown | 9 | 201 | |
| TNM stage | <0.001 | ||
| 0 | 12 | 199 | |
| 1 | 20 | 964 | |
| 2 | 17 | 1,348 | |
| 3 | 8 | 700 | |
| Unknown | 16 | 507 | |
| Status | 0.281 | ||
| Alive | 67 | 3,494 | |
| Death | 6 | 224 | |
2+: immunohistochemistry indicated a staining strength of 2+, but no fluorescence in situ hybridization test results were available. CPM, contralateral prophylactic mastectomy; UM, unilateral mastectomy; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis.
Trends in breast surgery procedures
Figure 2 demonstrated the results of statistical analysis of the trends in the number and proportion of breast surgeries for UBC patients from 2005 to 2017. Since 2005, the total number of surgeries had an overall upward trend each year and remained basically above 300 after 2009. The largest number of surgeries was performed in 2014 with a total of 527, followed by a decline to 298 in 2016.

CPM was first performed in 2007, and the rate increased from 0.40% to 3.02% in the period from 2008 to 2016 (P<0.001) but decreased to 1.05% in 2017. The SM rate between 2005 and 2017 increased from 69.4% to 82.5% (P<0.001), along with a decrease in the BCS rate from 30.6% to 12.3% (P<0.001), reaching a lowest of 6.50% in 2013. NSM was also first conducted in 2007, rising from 1.8% in 2007 to 4.2% in 2017 (P=0.004), with a maximum percentage of 7.36% in 2012.
Impact of CPM and other prognostic factors on survival
Survival analysis was performed with 3,791 patients in the CPM and UM groups. The median follow-up time was 66.60 months. There were 6 deaths (8.2%) in the CPM group and 224 deaths (6.0%) in the UM group. The 5- and 10-year OS were 94.52% and 93.15% for CPM group compared with 96.64% and 94.89% for UM group. In KM survival analysis (Figure 3), CPM patients had not demonstrated a better OS compared to UM patients (P=0.963).

Univariate and multivariate Cox regression hazard analyses were conducted to identify prognostic factors associated with OS (Table 2). The factors independently associated with improved OS included younger age, premenopausal status, ER and PR positive, lower tumor grade, and lower TNM stage. And the PR status (HR =0.409; 95% CI: 0.249–0.672; P<0.001) and TNM stage (HR =5.231; 95% CI: 3.016–9.073; P<0.001) were independent prognostic factors for survival and the remaining factors were not significant on multivariate analysis.
Table 2
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Group | |||||
| UM | 1 (reference) | – | – | ||
| CPM | 0.979 (0.403–2.380) | 0.963 | – | – | |
| Age (years) | 0.003 | 0.128 | |||
| <45 | 1 (reference) | 1 (reference) | |||
| ≥45–<60 | 0.935 (0.675–1.296) | 0.688 | 0.948 (0.568–1.583) | 0.839 | |
| ≥60 | 1.664 (1.157–2.395) | 0.006 | 1.595 (0.824–3.087) | 0.166 | |
| BMI (kg/m2) | 0.438 | ||||
| <18.5 | 1 (reference) | – | – | ||
| ≥18.5–<24 | 1.118 (0.410–3.050) | 0.827 | – | – | |
| ≥24–<28 | 1.333 (0.488–3.641) | 0.574 | – | – | |
| ≥28 | 1.512 (0.535–4.275) | 0.436 | – | – | |
| Menopausal state | |||||
| Premenopausal | 1 (reference) | 1 (reference) | |||
| Postmenopausal | 1.571 (1.140–2.166) | 0.006 | 1.264 (0.751–2.128) | 0.378 | |
| Family history of breast cancer | |||||
| No | 1 (reference) | – | – | ||
| Yes | 0.435 (0.139–1.362) | 0.153 | – | – | |
| Family history of other malignancies | |||||
| No | 1 (reference) | – | – | ||
| Yes | 0.672 (0.374–1.204) | 0.182 | – | – | |
| Contralateral breast events | 0.098 | ||||
| 0 | 1 (reference) | – | – | ||
| 2 | 0.617 (0.086–4.434) | 0.632 | – | – | |
| 3 | 0.353 (0.154–0.809) | 0.014 | – | – | |
| 4 | 1.063 (0.390–2.899) | 0.905 | – | – | |
| Surgery | |||||
| UM | 1 (reference) | – | – | ||
| NSM | 0.722 (0.339–1.535) | 0.397 | – | – | |
| ER | |||||
| Negative | 1 (reference) | 1 (reference) | |||
| Positive | 0.596 (0.443–0.802) | 0.001 | 1.018 (0.618–1.677) | 0.943 | |
| PR | |||||
| Negative | 1 (reference) | 1 (reference) | |||
| Positive | 0.431 (0.322–0.577) | <0.001 | 0.409 (0.249–0.672) | <0.001 | |
| HER2 | 0.763 | ||||
| Negative | 1 (reference) | – | – | ||
| Positive | 1.149 (0.780–1.692) | 0.482 | – | – | |
| 2+ | 1.084 (0.770–1.526) | 0.643 | – | – | |
| Ki67 | 0.298 | ||||
| Low | 1 (reference) | – | – | ||
| Medial | 1.268 (0.783–2.055) | 0.334 | – | – | |
| High | 1.420 (0.907–2.224) | 0.126 | – | – | |
| Grade | 0.015 | 0.817 | |||
| 1 | 1 (reference) | 1 (reference) | |||
| 2 | 6.081 (0.847–43.675) | 0.073 | 6,163.550 (0–9.249E+043) | 0.853 | |
| 3 | 8.721 (1.209–62.898) | 0.032 | 5,450.742 (0–8.182E+043) | 0.855 | |
| T stage | <0.001 | ||||
| 0 | 1 (reference) | – | – | ||
| 1 | 3.625 (0.883–14.875) | 0.074 | – | – | |
| 2 | 7.930 (1.954–32.183) | 0.004 | – | – | |
| 3 | 25.170 (5.995–105.677) | <0.001 | – | – | |
| N stage | <0.001 | ||||
| 0 | 1 (reference) | – | – | ||
| 1 | 2.121 (1.420–3.169) | <0.001 | – | – | |
| 2 | 3.646 (2.400–5.541) | <0.001 | – | – | |
| 3 | 10.326 (7.019–15.192) | <0.001 | – | – | |
| TNM stage | <0.001 | <0.001 | |||
| 0 | 1 (reference) | – | – | ||
| 1 | 2.320 (0.547–9.840) | 0.254 | 1 (reference) | ||
| 2 | 4.486 (1.096–18.368) | 0.037 | 1.644 (0.928–2.912) | 0.088 | |
| 3 | 15.330 (3.775–62.259) | <0.001 | 5.231 (3.016–9.073) | <0.001 | |
2+: immunohistochemistry indicated a staining strength of 2+, but no fluorescence in situ hybridization test results were available. OS, overall survival; HR, hazard ratio; CI, confidence interval; UM, unilateral mastectomy; CPM, contralateral prophylactic mastectomy; BMI, body mass index; NSM, nipple-sparing mastectomy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis.
Post-PSM analysis
With PSM, the match cohort obtained consisted of 64 patients receiving CPM and 64 patients receiving UM. The PSM results are summarized in Figure S1. No significant differences were observed in the analysis of clinicopathological factors and mortality between the matched two groups (Table S1). And the KM survival analysis result revealed no statistically significant difference in OS (P=0.834) and DFS (P=0.678) (Figure 4).

Univariate Cox regression hazard analyses (Table 3) showed no survival benefit in terms of better OS (HR =0.979; 95% CI: 0.403–2.380; P=0.963) and DFS (HR =0.922; 95% CI: 0.629–1.352; P=0.678) in CPM patients. The association between BMI and OS was significant in the matched cohort while other clinical or pathological factors significantly associated with OS and DFS were not found.
Table 3
| Factors | OS | DFS | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Group | 0.834 | 0.678 | |||
| UM | 1 (reference) | 1 (reference) | |||
| CPM | 0.876 (0.253–3.034) | 0.922 (0.629–1.352) | |||
| Age (years) | 0.335 | 0.409 | |||
| <45 | 1 (reference) | 1 (reference) | |||
| ≥45 | 0.514 (0.133–1.989) | 0.719 (0.329–1.573) | |||
| BMI (kg/m2) | 0.032 | 0.561 | |||
| <18.5 | 1 (reference) | 1 (reference) | |||
| ≥18.5–<24 | 0.006 (0.021–0.526) | 0.006 | 0.451 (0.128–1.590) | 0.216 | |
| ≥24–<28 | 0.151 (0.030–0.750) | 0.021 | 0.452 (0.122–1.678) | 0.236 | |
| ≥28 | 0.178 (0.018–1.712) | 0.135 | 0.314 (0.052–1.878) | 0.204 | |
| Menopausal state | 0.469 | 0.326 | |||
| Premenopausal | 1 (reference) | 1 (reference) | |||
| Postmenopausal | 0.440 (0.055–3.525) | 0.543 (0.160–1.838) | |||
| Family history of breast cancer | 0.469 | 0.223 | |||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 0.042 (0.000–228.955) | 0.289 (0.039–2.128) | |||
| Family history of other malignancies | 0.724 | 0.595 | |||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 1.450 (0.184–11.459) | 0.581 (0.078–4.308) | |||
| Contralateral breast events | 0.287 | 0.074 | |||
| 0 | 1 (reference) | 1 (reference) | |||
| BI-RADS 2/3/4 | 0.024 (0.000–23.228) | 0.304 (0.082–1.122) | |||
| ER | 0.235 | 0.760 | |||
| Negative | 1 (reference) | 1 (reference) | |||
| Positive | 0.420 (0.100–1.758) | 1.218 (0.344–4.308) | |||
| PR | 0.229 | 0.089 | |||
| Negative | 1 (reference) | 1 (reference) | |||
| Positive | 0.427 (0.107–1.708) | 0.431 (0.164–1.136) | |||
| HER2 | 0.285 | 0.554 | |||
| Negative | 1 (reference) | 1 (reference) | |||
| Positive | 1.954 (0.428–8.926) | 0.387 | 1.469 (0.436–4.945) | 0.535 | |
| 2+ | 0.369 (0.084–2.511) | 0.369 | 0.728 (0.254–2.083) | 0.554 | |
| Ki67 | 0.329 | 0.563 | |||
| Low | 1 (reference) | 1 (reference) | |||
| Medial | 0.169 (0.015–1.869) | 0.147 | 0.918 (0.177–4.753) | 0.919 | |
| High | 0.714 (0.138–3.688) | 0.688 | 1.595 (0.347–7.326) | 0.549 | |
| Grade | 0.417 | 0.372 | |||
| 1/2 | 1 (reference) | 1 (reference) | |||
| 3 | 1.974 (0.382–10.188) | 1.676 (0.539–5.206) | |||
| TNM stage | 0.138 | 0.662 | |||
| 0/1 | 1 (reference) | 1 (reference) | |||
| 2/3 | 2.789 (0.720–10.804) | 1.205 (0.522–2.783) | |||
2+: immunohistochemistry indicated a staining strength of 2+, but no fluorescence in situ hybridization test results were available. OS, overall survival; DFS, disease-free survival; OR, odds ratio; CI, confidence interval; UM, unilateral mastectomy; CPM, contralateral prophylactic mastectomy; BMI, body mass index; BI-RADS, Breast Imaging Reporting and Data System; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis.
Subgroup analysis
To further explore the specific patient subgroups that may derive survival benefits from CPM, a stratification analysis of the relationship between CPM and prognosis based on patient clinical and pathological factors was performed. The results of univariate Cox regression hazard analyses were plotted as forest plots (Figure 5). Limited by sample size and with reference to pre-PSM analysis and previous study findings, patients in subgroups age ≥45 years, BMI <24 kg/m2, BMI ≥24 kg/m2, T stage 0/1, T stage 2/3, and N stage 1/2/3 were combined, respectively. The results failed to show survival benefit in terms of OS and DFS in any subgroup of CPM patients.
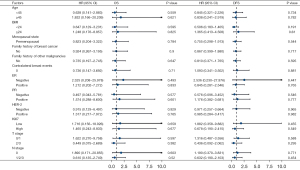
KM survival curves of each subgroup are shown in Figure S2. In terms of OS, with the subgroup of clinical factors, including age <45 years, BMI <24 kg/m2, and no contralateral breast events, survival appeared to be better in the CPM group than in the non-CPM group. A similar phenomenon was observed in the subgroups with pathological factors including T stage 2/3 and N stage 1/2/3. To eliminate the interaction between these factors, individuals with age <45 years, BMI <24 kg/m2, and no contralateral breast events were combined into subgroup 1, whereas T stage 2/3 and N stage 1/2/3 were combined into subgroup 2. However, the results of the Cox analysis showed that the CPM group remained without survival benefit (subgroup 1: HR =1.462, 95% CI: 0.130–16.467, P=0.758; subgroup 2: HR =0.340, 95% CI: 0.057–2.803, P=0.246). In terms of DFS, there was no survival difference between the two treatment groups as well.
Predictors of receiving CPM vs. UM
Univariate logistic regression analyses (Table 4) showed CPM was negatively associated with older age (≥45–<60 years: OR =0.374, 95% CI: 0.225–0.622, P<0.001; ≥60 years: OR =0.253, 95% CI: 0.107–0.597, P=0.002), higher BMI (≥24–<28 kg/m2: OR =0.244, 95% CI: 0.089–0.669, P=0.006; ≥28 kg/m2: OR =0.173, 95% CI: 0.049–0.610, P=0.006), postmenopausal (OR =0.380; 95% CI: 0.170–0.850; P=0.018) and higher TNM stage (stage 1: OR =0.344, 95% CI: 0.166–0.715, P=0.004; stage 2: OR =0.209, 95% CI: 0.098–0.444, P<0.001; stage 3: OR =0.190, 95% CI: 0.076–0.490, P<0.001).
Table 4
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (years) | <0.001 | 0.942 | |||
| <45 | 1 (reference) | 1 (reference) | |||
| ≥45–<60 | 0.374 (0.225–0.622) | <0.001 | 0.836 (0.301–2.321) | 0.731 | |
| ≥60 | 0.253 (0.107–0.597) | 0.002 | 0.000 (0.000–) | 0.994 | |
| BMI (kg/m2) | 0.006 | 0.444 | |||
| <18.5 | 1 (reference) | 1 (reference) | |||
| ≥18.5–<24 | 0.456 (0.177–1.179) | 0.105 | 0.320 (0.059–1.748) | 0.188 | |
| ≥24–<28 | 0.244 (0.089–0.669) | 0.006 | 0.604 (0.109–3.353) | 0.564 | |
| ≥28 | 0.173 (0.049–0.610) | 0.006 | 0.000 (0.000–) | 0.995 | |
| Menopausal state | |||||
| Premenopausal | 1 (reference) | 1 (reference) | |||
| Postmenopausal | 0.380 (0.170–0.850) | 0.018 | 1.597 (0.386–6.612) | 0.518 | |
| Family history of breast cancer | |||||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 6.228 (3.332–11.642) | <0.001 | 1.958 (0.389–9.851) | 0.415 | |
| Family history of other malignancies | |||||
| No | 1 (reference) | – | – | ||
| Yes | 0.795 (0.342–1.845) | 0.593 | – | – | |
| Contralateral breast events | 0.001 | 0.056 | |||
| 0 | 1 (reference) | 1 (reference) | |||
| 2 | 3.623 (0.829–15.832) | 0.087 | 0.000 (0.000–) | 0.998 | |
| 3 | 2.952 (1.519–5.738) | 0.001 | 3.716 (1.367–10.101) | 0.010 | |
| 4 | 4.155 (1.552–11.122) | 0.005 | 3.773 (0.726–19.623) | 0.114 | |
| Surgery | |||||
| UM | 1 (reference) | 1 (reference) | |||
| NSM | 21.758 (13.367–35.416) | <0.001 | 16.951 (6.344–45.290) | <0.001 | |
| T stage | 0.002 | ||||
| 0 | 1 (reference) | – | – | ||
| 1 | 0.321 (0.161–0.641) | 0.001 | – | – | |
| 2 | 0.254 (0.121–0.530) | <0.001 | – | – | |
| 3 | 0.212 (0.047–0.960) | 0.044 | – | – | |
| N stage | 0.579 | ||||
| 0 | 1 (reference) | – | – | ||
| 1 | 0.713 (0.380–1.337) | 0.292 | – | – | |
| 2 | 0.644 (0.272–1.526) | 0.317 | – | – | |
| 3 | 0.716 (0.254–2.015) | 0.527 | – | – | |
| TNM stage | <0.001 | 0.049 | |||
| 0 | 1 (reference) | 1 (reference) | |||
| 1 | 0.344 (0.166–0.715) | 0.004 | 0.343 (0.097–1.213) | 0.097 | |
| 2 | 0.209 (0.098–0.444) | <0.001 | 0.148 (0.037–0.587) | 0.007 | |
| 3 | 0.190 (0.076–0.470) | <0.001 | 0.214 (0.044–1.048) | 0.057 | |
CPM, contralateral prophylactic mastectomy; OR, odds ratio; CI, confidence interval; BMI, body mass index; UM, unilateral mastectomy; NSM, nipple-sparing mastectomy; TNM, tumor-node-metastasis.
Patients who had a family history of breast cancer (OR =6.228; 95% CI: 3.332–11.642; P<0.001) were significantly more likely to receive a CPM, as well as the surgical procedure of NSM (OR =21.758; 95% CI: 13.367–35.416; P<0.001) and contralateral breast events (BI-RADS 3: OR =2.952, 95% CI: 1.519–5.738, P=0.001; BI-RADS 4: OR =4.155, 95% CI: 1.552–11.122, P=0.005). No association was noted between CPM and the family history of other malignancies.
In the multivariate logistic regression analyses, it was founded that the surgical procedure of NSM (OR =17.576; 95% CI: 6.757–45.719; P=0.018) and lower TNM stage (stage 1: OR =0.343, 95% CI: 0.097–1.213, P=0.097; stage 2: OR =0.148, 95% CI: 0.037–0.587, p=0.007; stage 3: OR =0.214, 95% CI: 0.044–1.048, P=0.057) were closely associated with CPM (OR =17.576; 95% CI: 6.757–45.719; P=0.018) and the remaining factors were not significant.
Postoperative satisfaction analysis
Postoperative satisfaction was followed up in 73 CPM patients, and the results are shown in Table S2; 42.5% of the patients’ CPM surgery was recommended by the doctors and 38.4% of the patients’ surgical decisions were made by the patients themselves or their families; 71.2% of the patients were completely satisfied with the overall postoperative situation, 13.7% of the patients expressed basic satisfaction, and only 2 patients (2.7%) regretted undergoing the CPM, mainly associated with poor satisfaction in physical appearance. In terms of satisfaction with physical appearance, only 32.9% of the patients were completely satisfied, 37.0% were basically satisfied, and 16.4% gave a poor evaluation of the cosmetic effect.
Considering that patients’ cosmetic requirements may be related to the choice of surgical CPM procedure (SM or NSM) and whether breast reconstruction was performed, a univariate logistic regression analyses was performed, while the results were not statistically significant (Table 5).
Table 5
| Factors | OR (95% CI) | P value |
|---|---|---|
| Surgery | ||
| SM | 1 (reference) | |
| NSM | 2.111 (0.504–8.843) | 0.307 |
| Breast reconstruction | ||
| No | 1 (reference) | |
| Yes | 1.778 (0.475–6.656) | 0.393 |
CPM, contralateral prophylactic mastectomy; OR, odds ratio; CI, confidence interval; SM, simple mastectomy; NSM, nipple-sparing mastectomy.
In the impact on quality of life, 8.2% of patients happened upper limb edema after surgery; 27.4% of patients reported that labor was affected to varying degrees, while about 10% of patients suggested that emotional life, work situation, social activities, and daily exercise were affected by surgery, respectively; 53.4% of patients refused to answer the impact of CPM surgery on sexual life, and 6.8% of patients reported that sexual life was affected mildly or moderately (Table S2).
Discussion
CPM is a controversial but popular topic worldwide. Although there is insufficient evidence that CPM can significantly improve survival outcomes in breast cancer patients with general CBC risk, most female UBC patients tend to make CPM surgical decisions due to concerns about the risk of recurrence and the pursuit of breast cosmetic symmetry. Based on a Chinese patient cohort, this study analyzed and summarized CPM application trends, decision-making related factors, impact on survival outcomes such as OS and DFS, postoperative satisfaction, and impact on life quality in order to provide a reference for the future clinician and patient decisions on CPM procedures.
The results of the application trend analysis showed that CPM was first performed at our hospital in 2007, nearly 30 years later than in the 1970s in Europe and the United States. The proportion of CPM procedures as a percentage of total annual surgical procedures in UBC patients at our hospital increased significantly between 2005 and 2017 (P<0.001), with the rate increasing from 0.40% to 3.02% between 2008 and 2016. This is consistent with the growth trend from 3.9% to 12.7% from 2002 to 2012 in the SEER database but with a lower contribution. The increase in CPM application rates, especially the rapid increase after 2012, was considered to be related to the celebrity effect of media coverage, known as the “Angelina Jolie” effect (22,23). In 2017, the number of CPMs dropped precipitously and were not performed thereafter primarily due to the ASBrS guidelines did not recommend CPM surgery for UBC patients at general CBC risk, so we followed and reduced. Overall, the CPM application rate in the Chinese patient cohort is much lower than that in the Western population, which is mainly considered to be related to the more conservative personality characteristics of Chinese people. The patients are reluctant to choose more invasive procedures and undertake heavier medical burdens while the doctors consider the procedure to bring higher medical risks.
Despite the current increase in the proportion of female UBC patients choosing CPM, there is still a lack of validated evidence that CPM improves long-term survival in average-risk patients. Several large cohort studies have shown no significant advantage of CPM over BCS in terms of BCSS and OS (1,3,24). Similar results were obtained in this study. There was no significant improvement in OS in the CPM group compared with the UM group in the KM survival curve analysis (P=0.963) and CPM neither show a significant correlation with improved OS in the univariate Cox regression hazard analyses (P=0.834), either before or after PSM. The results suggest that CPM could not lead to a better survival outcome for the Chinese female UBC patients.
It has been suggested that CPM was associated with improved 5-year BCSS in specific patient subgroups (25). Risk-stratified analysis showed that this association was because of a reduction in breast cancer-specific mortality in women aged 18–49 years with stages I–II ER-negative cancer (HR =0.68; 95% CI: 0.53–0.88; P=0.004). Likewise, Fayanju et al. (16) suggested that the OS benefit of CPM may be influenced by selection bias, as CPM recipients were more likely to have characteristics associated with improved survival, in terms of early tumors and adequate Medicare coverage. Our study also explored other clinicopathologic factors that may influence patients’ survival outcomes with stratified subgroup analysis in the post-PSM cohort. However, no survival benefit was observed in all age, BMI, premenopausal, family history of breast cancer and other malignancies, contralateral breast events, ER, PR, HER2, Ki67, T-stage, and N-stage CPM subgroups. Combining subgroups of patients aged <45 years with a BMI <24 kg/m2 and no contralateral breast events, and subgroups of T stage 2/3 and N stage 1/2/3, neither showed an improvement in OS with CPM.
Patients with greater awareness of tumor risk have an intense fear of the disease and are willing to choose the more invasive CPM instead of BCS (26,27). Although it has been demonstrated that CPM can reduce the risk of developing CBC by approximately 90% in females with genetic susceptibilities, such as BRCA carrier status and/or family history of breast cancer, but not in the general breast cancer population (16,28,29). In previous studies, the surgical decision for CPM was influenced by BRCA1/2 mutations and a family history of malignancy (30,31). Our study also found that patients with a family history of breast cancer were preferred for CPM surgery, as well as the patients with contralateral breast lesions of BI-RADS category 3 or 4. This phenomenon is consistent with strong concerns about disease recurrence and CBC risk. As for BRCA genetic testing, it is not routinely performed due to high cost. In addition, patients choosing the NSM surgical procedure were more likely to undergo CPM compared to the SM, with considerations related to the greater desire for physical appearance.
The patients were followed up for postoperative satisfaction and quality of life; 69.9% of patients expressed satisfaction with their postoperative appearance, but the NSM procedure and postoperative breast reconstruction showed no higher satisfaction, probably linked to the high expectations for the cosmetic results of CPM. In terms of impact on quality of life, physical labor was most affected, likely associated with upper limb edema and a reduction in upper limb activity due to postoperative wound pain and fear of poor healing.
Presently, clinical recommendations of CPM are based on the presence of a pathogenic mutation in BRCA1/2 (32). Therefore, in patients at average risk for CBC, clinicians and patients should sufficiently discuss the decision for CPM, especially with respect to the increased risk of postoperative complications and the potential failure of postoperative breast morphology to meet expectations. Several risk prediction models, including the Manchester formula, CBCrisk, BOADICEA model, and PredictCBC, have been developed to calculate the risk of an individual developing CBC (33-37). It is promising to use them to tailor clinical decision-making toward CPM or alternative preventive strategies, but careful recalibration is required before clinical application.
This study had several limitations. First, it was a retrospective study conducted in a single center, therefore selection bias may occur. Second, the sample in the CPM group was small and follow-up was limited. In addition, more detailed information such as BRCA 1/2 mutations and the utilization of preoperative neoadjuvant therapy and postoperative adjuvant therapy was lacking, which may influence the surgical options and survival outcomes of patients.
Conclusions
This study suggested that CPM was practiced for the first time in our Chinese medical institutions since 2007, with the highest rate in 2016, and subsequently declined and is rarely used now. CPM was not an independent prognostic factor for OS compared to UM. The patients with lower TNM stage and NSM surgical procedure preferred to perform CPM. The appearance satisfaction with CPM procedure was low and a significant percentage of patients expressed limitations in physical functioning. Therefore, clinicians should be more cautious in advising patients on CPM and providing adequate reference information, especially about the impact on survival and breast appearance of CPM, to guide patients to make more rational surgical decisions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-384/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-384/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-384/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-384/coif). X.L. serves as an Editor-in-Chief of Gland Surgery from May 2022 to April 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of The First Medical Center of Chinese PLA General Hospital (No. S2020-451-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA 2014;312:902-14. [Crossref] [PubMed]
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [Crossref] [PubMed]
- Wong SM, Freedman RA, Sagara Y, et al. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017;265:581-9. [Crossref] [PubMed]
- Shaheen MS, Momeni A. Nationwide Trends in Contralateral Prophylactic Mastectomies: An Analysis of 55,060 Unilateral Breast Cancer Patients. Plast Reconstr Surg Glob Open 2022;10:e4344. [Crossref] [PubMed]
- Sim Y, Tan VK, Ho GH, et al. Contralateral prophylactic mastectomy in an Asian population: a single institution review. Breast 2014;23:56-62. [Crossref] [PubMed]
- Brazilian Medical Association. Contralateral prophylactic mastectomy. Rev Assoc Med Bras (1992) 2018;64:3-8. [Crossref] [PubMed]
- Carbine NE, Lostumbo L, Wallace J, et al. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev 2018;4:CD002748. [Crossref] [PubMed]
- Chung A, Huynh K, Lawrence C, et al. Comparison of patient characteristics and outcomes of contralateral prophylactic mastectomy and unilateral total mastectomy in breast cancer patients. Ann Surg Oncol 2012;19:2600-6. [Crossref] [PubMed]
- Boccardo C, Gentilini O. Contralateral risk reducing mastectomy in patients with sporadic breast cancer. Benefits and hazards. Eur J Surg Oncol 2016;42:913-8. [Crossref] [PubMed]
- Daly MB, Pal T, Berry MP, et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:77-102. [Crossref] [PubMed]
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017;317:2402-16. [Crossref] [PubMed]
- Boughey JC, Attai DJ, Chen SL, et al. Contralateral Prophylactic Mastectomy Consensus Statement from the American Society of Breast Surgeons: Additional Considerations and a Framework for Shared Decision Making. Ann Surg Oncol 2016;23:3106-11. [Crossref] [PubMed]
- Hawley ST, Jagsi R, Morrow M, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surg 2014;149:582-9. [Crossref] [PubMed]
- Ager B, Butow P, Jansen J, et al. Contralateral prophylactic mastectomy (CPM): A systematic review of patient reported factors and psychological predictors influencing choice and satisfaction. Breast 2016;28:107-20. [Crossref] [PubMed]
- Fairbairn K, Cervantes A, Rayhrer C, et al. Trends in Contralateral Prophylactic Mastectomy. Aesthetic Plast Surg 2020;44:323-9. [Crossref] [PubMed]
- Fayanju OM, Stoll CR, Fowler S, et al. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg 2014;260:1000-10. [Crossref] [PubMed]
- Chen C, Lu FC. Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 2004;17:1-36.
- Gradishar WJ, Anderson BO, Abraham J, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. [Crossref] [PubMed]
- Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol 2018;25:1783-5.
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [Crossref] [PubMed]
- Evans DG, Barwell J, Eccles DM, et al. The Angelina Jolie effect: how high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res 2014;16:442. [Crossref] [PubMed]
- Mao J, Jorm L, Sedrakyan A. Trends in Use of Risk-Reducing Mastectomy in a Context of Celebrity Decisions and Media Coverage: An Observational Study in the United States and Australia. Health Serv Res 2018;53:2682-95. [Crossref] [PubMed]
- Lazow SP, Riba L, Alapati A, et al. Comparison of breast-conserving therapy vs mastectomy in women under age 40: National trends and potential survival implications. Breast J 2019;25:578-84. [Crossref] [PubMed]
- Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst 2010;102:401-9. [Crossref] [PubMed]
- Portschy PR, Abbott AM, Burke EE, et al. Perceptions of Contralateral Breast Cancer Risk: A Prospective, Longitudinal Study. Ann Surg Oncol 2015;22:3846-52. [Crossref] [PubMed]
- Zheng Y, Li J, Hong C, et al. Clinical features, prognosis, and influencing factors of contralateral prophylactic mastectomy in 58 patients with breast cancer. Ann Transl Med 2020;8:1665. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055-62. [Crossref] [PubMed]
- Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med 2013;159:373-81. [Crossref] [PubMed]
- Fisher CS, Martin-Dunlap T, Ruppel MB, et al. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol 2012;19:3246-50. [Crossref] [PubMed]
- Basu NN, Ross GL, Evans DG, et al. The Manchester guidelines for contralateral risk-reducing mastectomy. World J Surg Oncol 2015;13:237. [Crossref] [PubMed]
- Chowdhury M, Euhus D, Onega T, et al. A model for individualized risk prediction of contralateral breast cancer. Breast Cancer Res Treat 2017;161:153-60. [Crossref] [PubMed]
- Chowdhury M, Euhus D, Arun B, et al. Validation of a personalized risk prediction model for contralateral breast cancer. Breast Cancer Res Treat 2018;170:415-23. [Crossref] [PubMed]
- Giardiello D, Steyerberg EW, Hauptmann M, et al. Prediction and clinical utility of a contralateral breast cancer risk model. Breast Cancer Res 2019;21:144. [Crossref] [PubMed]
- Giardiello D, Hooning MJ, Hauptmann M, et al. PredictCBC-2.0: a contralateral breast cancer risk prediction model developed and validated in ~ 200,000 patients. Breast Cancer Res 2022;24:69. [Crossref] [PubMed]
- Giardiello D, Hauptmann M, Steyerberg EW, et al. Prediction of contralateral breast cancer: external validation of risk calculators in 20 international cohorts. Breast Cancer Res Treat 2020;181:423-34. [Crossref] [PubMed]


