
Diagnostic performance of contrast-enhanced ultrasound in breast lesions: what diagnostic models could be used for lesions of different sizes?
Highlight box
Key findings
• The diagnostic efficiencies of contrast-enhanced ultrasound (CEUS) for lesions of different sizes varied.
• New models were established for lesions of different sizes.
• Diagnostic model for lesions ≤1.0 cm was different from that for other lesions.
What is known and what is new?
• The diagnostic value of CEUS had a higher accuracy for lesions >2.0 cm.
• Diagnostic model for lesions ≤1.0 cm was different from that for other lesions.
What is the implication, and what should change now?
• CEUS is effective for the diagnosis of small breast lesions and different models are required for breast lesions of different sizes.
Introduction
Ultrasound (US) is widely used for breast check-up due to its wide availability, low price, and patient acceptance. In addition to conventional US, the development of contrast-enhanced ultrasound (CEUS) allows evaluation of microvascular structures, density, and blood perfusion of tumors, offering more valuable information about lesions (1). Previous studies have confirmed the value of CEUS in the diagnosis of benign and malignant breast lesions (2-4). However, there are some limitations in applying CEUS to the breast and there is a lack of uniform diagnostic criteria. In a previous study, size of lesions was shown to affect the diagnostic accuracy of CEUS. The diagnostic value of CEUS varied with the size of lesions and it had a higher accuracy for lesions >2.0 cm (5). Tumor angiogenesis varies with tumor size (6). In addition, tumor size is considered an important reference point for breast cancer treatment, as well as a strong predictor of prognosis (7). However, it is unclear whether CEUS has good diagnostic performance for lesions ≤1.0 and ≤2.0 cm. For early diagnosis, it is beneficial to explore the application of CEUS in breast lesions of different sizes. In this study, the lesions were divided into three groups according to their size to analyze the diagnostic performance of CEUS in breast lesions of different sizes and to explore more models better applied to these lesions. We present this article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-223/rc).
Methods
Patients selected
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective clinical diagnostic study was approved by the Ethics Committee of the Chinese PLA General Hospital (No. S2021-683-01). Informed consent was waived because of the retrospective nature of the study. The outcome of interest in this study was binary diagnosis of benign and malignant lesions. Between January 2021 and April 2022, 1,059 breast lesions from 1,059 consecutive patients who underwent US and CEUS at the General Hospital of the People’s Liberation Army of China were retrospectively examined in this study. The inclusion criteria were as follows: (I) patients with newly found solid breast lesions; (II) patients with breast lesions examined by both US and CEUS successively; and (III) patients with definite pathological findings. The exclusion criteria were as follows: (I) patients who received radiotherapy and chemotherapy; (II) patients with a history of breast surgery; (III) incomplete or unable to accurately analyze the image information. The flowchart of patient selection is shown in Figure 1. Using histopathological results of the surgical or vacuum-assisted excisional specimens as the diagnostic standard, 654 malignant and 405 benign lesions were identified. These lesions were divided into three groups according to size (Group 1: ≤1.0 cm; Group 2: >1.0–2.0 cm, Group 3: >2.0 cm).
US equipment
All sonographic examinations were performed by ACUSON S2000 US system (Siemens, Erlangen, Germany) with a 9L4 linear array probe. The contrast agent used for CEUS imaging was SonoVue (Bracco International, Milan, Italy), a sterile freeze-dried powder consisting of phospholipid microbubbles, which can be metabolized through respiration within 30 min.
US examination
Both conventional US and CEUS examinations were performed by two physicians with more than 5 years of experience in breast ultrasonography. Once a lesion was detected, its images obtained in different planes were stored and its size was recorded. The size was defined as the greatest dimension of tumor measured using conventional US. If a patient had multiple lesions, the one with the largest diameter was included in this study. Subsequently, the system was switched to CEUS mode, and the mechanical index was set as 0.06–0.08. The optimal plane for CEUS showed both the largest diameter and sufficient surrounding normal tissue. A bolus of 5.0 mL of contrast agent was injected into an antecubital vein, followed by a flush of 5.0–10.0 mL of saline. Applying dual-screen mode, conventional US and CEUS images of the same plane were displayed in real time for observation and comparison. The lesion and surrounding tissues were recorded as real time images and a 3-min-lasting video for follow-up analyses. If the same patient required reexamined by CEUS, the interval was >20 min.
US and CEUS image analyses
All conventional US and CEUS images were analyzed independently by two physicians blinded to clinical information and final pathological results. Final decisions were made by consensus. According to the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) (8), lesions examined using conventional US were divided into five categories: 3, 4A, 4B, 4C, and 5.
According to CEUS performance, all lesions were evaluated for the following features:
- Enhancement direction (centripetal or centrifugal/diffuse/absent).
- Enhancement degree: enhanced intensity compared with surrounding breast tissue (hyper-/iso-/nonenhancement or hypo-enhancement).
- Enhancement homogeneity: internal homogeneity of the lesion at the peak of enhancement (homogeneous or heterogeneous).
- Blood perfusion defects: the area without microbubble contrast media perfusion (present or absent).
- Enhancement scope: comparison of the maximum diameter in CEUS images with two-dimensional images (enlarged or not enlarged).
- Radial or penetrating vessels: radial enhancement around the lesion (present or absent).
- Enhancement shape (regular or irregular).
- Wash-in time: entered time compared with surrounding breast tissue (earlier or synchronous/later/absent).
- Wash-out time: withdrew time compared with surrounding breast tissue (earlier/synchronous/absent or later).
When the enhancement degree of a lesion was non-enhancement, the enhancement direction, wash-in time, and wash-out time of the lesion were defined as absence.
Sample size calculation
The sample size was calculated on the basis of literature review (5). The sensitivity and specificity of CEUS for breast lesions were 0.76 and 0.86, respectively. The power (1-β) was 0.90, and allowed error was 0.1. Considering the loss of follow-up rate of 10%, the study should include 546 benign and 219 malignant lesions, for a total of 765 cases.
Statistical analysis
Statistical analyses were performed using SPSS 25.0 (SPSS Inc, Chicago, IL, USA) MedCalc 20 (MedCalc Software Ltd, Ostend, Belgium) and PASS 15.0 (PASS Software, Rijswijk, The Netherlands). Patients who underwent US and CEUS between January 2021 and December 2021 were included in the training set, and patients who underwent US and CEUS between January 2022 and April 2022 were included in the validation set. Independent t-test was used for normally distributed data, whereas Mann-Whitney U test was used for non-normally distributed data. Diagnostic models were established using multivariate binary logistic regression analyses of CEUS features from the training set. Diagnostic performance was evaluated using the area under the receiver operating characteristic curve (AUC) and validated in the validation set. When combining diagnostic models and BI-RADS, if the CEUS model result was positive, the original BI-RADS score was raised one level. If negative, the original BI-RADS score was downgraded one level. After the receiver operating characteristic (ROC) curve was operated, AUC values were calculated, and Youden’s index was used to calculate the optimal cutoff values. The cutoff value divided the samples into positive and negative, and then sensitivity and specificity were calculated. The DeLong test was used to compare the ROC curves. Two-sided P values of <0.05 were considered indicative of statistically significant differences.
Results
Basic information of patients and lesions
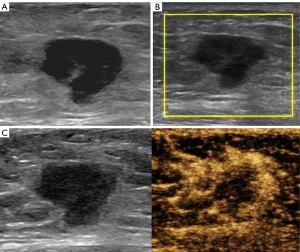
All 1,059 patients were women aged 18–80 years, with a mean age of 45±11 years. All lesions had a mean size of 1.8±1.2 cm (range, 0.2–13.9 cm). A total of 847 lesions (80.0%) were included in the training set, and 212 (20.0%) were included in the validation set. Patient age, lesion size, and pathological types of training and validation sets are listed in Table 1. All lesions in the training set were divided into three groups. Groups 1 (Figure 2), 2 (Figure 3), and 3 (Figure 4) with 208, 362, and 277 lesions, respectively. No severe adverse events were reported during conventional US and CEUS examinations.
Table 1
| Variables | Training set | Validation set |
|---|---|---|
| Age (years), mean ± standard deviation | 45±11 | 45±12 |
| Lesion size (cm), mean ± standard deviation | 1.9±1.3 | 1.5±0.9 |
| Histological benign, n (%) | n=503 | n=153 |
| Fibroadenoma | 238 (47.3) | 49 (32.0) |
| Mastopathy | 214 (42.5) | 61 (39.9) |
| Intraductal papilloma | 25 (5.0) | 31 (20.3) |
| Benign phyllodes tumor | 4 (0.8) | 1 (0.7) |
| Hamartoma | 1 (0.2) | 0 |
| Inflammation | 21 (4.2) | 11 (7.2) |
| Histological malignant, n (%) | n=344 | n=59 |
| Invasive carcinoma of no special type | 293 (85.2) | 42 (71.2) |
| Invasive lobular carcinoma | 6 (1.7) | 0 |
| Ductal carcinoma in situ | 37 (10.8) | 14 (23.7) |
| Mucinous carcinoma | 5 (1.5) | 1 (1.7) |
| Lobular carcinoma in situ | 1 (0.3) | 1 (1.7) |
| Medullary carcinoma | 1 (0.3) | 1 (1.7) |
| Diffuse large B-cell lymphoma | 1 (0.3) | 0 |


Logistic regression analyses of CEUS features of all lesions
The BI-RADS categories of the 847 lesions in the training set, based on conventional US are shown in Table 2. The sensitivity, specificity, accuracy and AUC of BI-RADS categories were 83.7%, 81.2%, 82.2% and 0.875, respectively.
Table 2
| BI-RADS category | Final pathologic results | Total | |
|---|---|---|---|
| Benign (n=503) | Malignant (n=344) | ||
| Category 3 | 108 | 3 | 111 |
| Category 4A | 300 | 53 | 353 |
| Category 4B | 79 | 117 | 196 |
| Category 4C | 15 | 95 | 110 |
| Category 5 | 1 | 76 | 77 |
| Total | 503 | 347 | 847 |
BI-RADS, Breast Imaging Reporting and Data System.
Univariate and multivariate regression analyses of CEUS features of all lesions in the training set are shown in Table 3. In univariate analysis, centripetal enhancement (P<0.001), hyperenhancement (P<0.001), heterogeneous enhancement (P<0.001), presence of blood perfusion defects (P<0.001), enlarged enhancement scope (P<0.001), presence of radial or penetrating vessels (P<0.001), irregular enhancement shape (P<0.001), ill-defined enhancement margin (P<0.001), earlier wash-in time (P<0.001) and later wash-out time (P<0.001) were associated with malignancy. All the aforementioned characteristics were subsumed in multivariate regression analysis. It showed that enhancement direction [odds ratio (OR) =2.31, P=0.001], blood perfusion defects (OR =1.72, P=0.028), scope (OR =5.45, P<0.001), radial or penetrating vessels (OR =2.30, P=0.001), shape (OR =1.96, P=0.031) and wash-out time (OR =1.94, P=0.003) were the independent influencing factors for malignant breast lesions.
Table 3
| CEUS features | Overall, n (%) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Benign (n=503) | Malignant (n=344) | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Enhancement direction | ||||||||
| Centrifugal/diffuse/absent | 229 (45.5) | 42 (12.2) | 1 (reference) | |||||
| Centripetal | 274 (54.5) | 302 (87.8) | 6.01 (4.16–8.67) | <0.001* | 2.31 (1.42–3.76) | 0.001* | ||
| Enhancement degree | ||||||||
| Iso/hypo/non-enhancement | 271 (53.9) | 47 (13.7) | 1 (reference) | |||||
| Hyperenhancement | 232 (46.1) | 297 (86.3) | 7.38 (5.18–10.51) | <0.001* | 1.02 (0.48–2.14) | 0.957 | ||
| Enhancement homogeneity | ||||||||
| Homogeneous | 194 (38.6) | 75 (21.8) | 1 (reference) | |||||
| Heterogeneous | 309 (61.4) | 269 (78.2) | 2.25 (1.64–3.07) | <0.001* | 1.11 (0.69–1.78) | 0.659 | ||
| Blood perfusion defects | ||||||||
| Absent | 433 (86.1) | 233 (67.7) | 1 (reference) | |||||
| Present | 70 (13.9) | 111 (32.3) | 2.94 (2.10–4.13) | <0.001* | 1.72 (1.06–2.79) | 0.028* | ||
| Enhancement scope | ||||||||
| Not enlarged | 428 (85.1) | 75 (21.8) | 1 (reference) | |||||
| Enlarged | 75 (14.9) | 269 (78.2) | 20.46 (14.35–29.17) | <0.001* | 5.45 (3.36–8.83) | <0.001* | ||
| Radial/penetrating vessels | ||||||||
| Absent | 466 (92.6) | 168 (48.8) | 1 (reference) | |||||
| Present | 37 (7.4) | 176 (51.2) | 13.19 (8.88–19.60) | <0.001* | 2.30 (1.39–3.81) | 0.001* | ||
| Enhancement shape | ||||||||
| Regular | 410 (81.5) | 85 (24.7) | 1 (reference) | |||||
| Irregular | 93 (18.5) | 259 (75.3) | 13.43 (9.63–18.73) | <0.001* | 1.96 (1.06–3.62) | 0.031* | ||
| Enhancement margin | ||||||||
| Well-defined | 392 (77.9) | 105 (30.5) | 1 (reference) | |||||
| Ill-defined | 111 (22.1) | 239 (69.5) | 8.03 (5.88–10.97) | <0.001* | 1.42 (0.79–2.54) | 0.238 | ||
| Wash-in time | ||||||||
| Synchronous/later/absent | 284 (56.5) | 53 (15.4) | 1 (reference) | |||||
| Earlier | 219 (43.5) | 291 (84.6) | 7.12 (5.05–10.02) | <0.001* | 1.00 (0.47–2.10) | 0.998 | ||
| Wash-out time | ||||||||
| Earlier/synchronous/absent | 311 (61.8) | 67 (19.5) | 1 (reference) | |||||
| Later | 192 (38.2) | 277 (80.5) | 6.69 (4.85–9.23) | <0.001* | 1.94 (1.25–3.01) | 0.003* | ||
*, statistical significance. CEUS, contrast-enhancement ultrasound; OR, odds ratio; CI, confidence interval.
M0: the fitting model of all lesions is as follows.
X1 indicates enhancement direction (centrifugal/diffuse/absent, 0; centripetal, 1). X2 indicates blood perfusion defects (iso/hypo/non-enhancement, 0; hyper-enhancement, 1). X3 indicates enhancement shape (regular, 0; irregular, 1). X4 indicates enhancement scope (not enlarged, 0; enlarged, 1). X5 indicates radial or penetrating vessels (absent, 0; present, 1). X6 indicates wash-out time (earlier/synchronous/absent, 0; later, 1).
In the training set, the sensitivity, specificity, accuracy, and AUC of the M0 combined with BI-RADS were 84.8%, 85.7%, 85.3%, and 0.921 respectively. The AUC value of M0 combined with BI-RADS in the validation set was 0.922. No statistical differences were found between the training and validation sets (0.921 vs. 0.922, P=0.893), indicating that M0 effectively diagnosed benign and malignant breast lesions in both datasets.
Comparison of the diagnostic performance of CEUS in three groups
M0 was highly efficient when applied in Groups 2 and 3, while it was far inferior in Group 1. By applying M0 into three groups and combining it with BI-RADS, the AUC values of three groups were 0.844, 0.936 and 0.928, respectively. M0 was less effective in Group 1 (0.844 vs. 0.921, P=0.029), but fitted well in Groups 2 and 3 (0.936 vs. 0.921, P=0.360; 0.928 vs. 0.921, P=0.711). More details are presented in Table 4 and the ROC curves of M0 applying for three groups are shown in Figure 5.
Table 4
| Group | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC | P value |
|---|---|---|---|---|---|
| Total (n=847) | 0.001* | ||||
| BI-RADS | 83.7 | 81.2 | 82.2 | 0.875 | |
| CEUS | 88.9 | 79.3 | 83.3 | 0.885 | |
| Combined | 84.8 | 85.7 | 85.3 | 0.921 | |
| ≤1.0 cm (n=208) | 0.004* | ||||
| BI-RADS | 60.9 | 85.1 | 79.4 | 0.752 | |
| CEUS | 76.1 | 85.8 | 83.5 | 0.796 | |
| Combined | 84.8 | 82.4 | 83.0 | 0.844 | |
| >1.0–2.0 cm (n=362) | <0.001* | ||||
| BI-RADS | 82.6 | 82.3 | 82.4 | 0.878 | |
| CEUS | 84.8 | 86.4 | 85.8 | 0.901 | |
| Combined | 92.8 | 81.4 | 85.8 | 0.936 | |
| >2.0 cm (n=277) | 0.007* | ||||
| BI-RADS | 91.2 | 73.9 | 84.0 | 0.899 | |
| CEUS | 84.9 | 79.1 | 82.5 | 0.883 | |
| Combined | 86.2 | 86.1 | 86.2 | 0.928 | |
*, statistical significance. AUC, area under the curve; BI-RADS, Breast Imaging Reporting and Data System; CEUS, contrast-enhancement ultrasound.

Logistic regression analyses of CEUS features of lesions with different sizes
Univariate and multivariate analyses of Groups 1–3 are subsumed in Figure 6 and detailed in Tables S1-S3.

In Group 1, there were statistically significant differences in enhancement direction (P=0.001), degree (P<0.001), scope (P<0.001), radial or penetrating vessels (P<0.001), shape (P<0.001), margin (P<0.001) and wash-in time (P<0.001) between benign and malignant lesions. All the above characteristics were subsumed in multivariate analysis, showing that scope was the independent influencing factor for malignant breast lesions (OR =2.710, P=0.001).
M1: the fitting model of group 1 is as follows:
X1 indicates direction (centrifugal/diffuse/absent, 0; centripetal, 1). X2 indicates wash-in time (synchronous/later/absent, 0; earlier, 1). X3 indicates degree (iso/hypo/non-enhancement, 0; hyper-enhancement, 1). X4 indicates shape (regular, 0; irregular = 1). X5 indicates margin (well-defined, 0; ill-defined, 1). X6 indicates scope (not enlarged, 0; enlarged, 1). X7 indicates radial or penetrating vessels (absent, 0; present = 1).
In the training set, the sensitivity, specificity, accuracy, and AUC of M1 combined with BI-RADS were 89.1%, 79.1%, 81.5% and 0.893 respectively. The AUC of M1 combined with BI-RADS in the validation set was 0.837. No statistical differences were noted between the training and validation sets (0.893 vs. 0.837, P=0.204), indicating that M1 was effective in diagnosing benign and malignant breast lesions ≤1.0 cm in both datasets. The diagnostic efficiency of M1 combined with BI-RADS was higher than that of M0 combined with BI-RADS (0.893 vs. 0.844, P=0.047) (Figure 7).

In the same way, M1 was established by Group 1, and so were the new models of Groups 2 and 3 performed. No statistical differences were noted between the diagnostic efficacy of the new models combined with BI-RADS established by Groups 2 and 3 and that of M0 combined with BI-RADS (0.937 vs. 0.936, P=0.243 and 0.934 vs. 0.928, P=0.246) (Figure 7).
Discussion
The diagnostic performance of CEUS varies for lesions of different sizes. Correct identification of these differences is vital for the diagnosis of breast lesions. Multivariate regression analysis showed that enhancement direction, wash-in time, degree, shape, margin, scope, and radial or penetrating vessels were independent factors associated with benign and malignant pathological results. A previous study had reported similar findings. Wan et al. (9) showed that enhancement degree, direction, homogeneity, margin, and radial or penetrating vessels could effectively distinguish between benign and malignant breast lesions.
During CEUS, the inflammatory response and the angiogenesis of malignant lesions are likely to cause enlarged enhancement scope, ill-defined margin and irregular shape, which play important roles in the diagnosis of benign and malignant breast lesions (10). Some studies showed that malignant tumors trigger local inflammatory responses that establish a primordial microenvironment (11,12). Tumor cells infiltrate cleavage products into surrounding normal tissues with the assistance of proteolytic enzymes and matrix degrading enzymes, stimulating angiogenesis in the surrounding area (13). More centripetal enhancement is observed in malignant lesions, whereas more centrifugal or diffuse enhancement is observed in benign ones. The direction of enhancement is related to tumor microvascular density (MVD) (14). In malignant tumors, the proportion of microvessels in peripheral region is higher than that in the central region, whereas in benign lesions, only slight differences are observed in the distribution (15,16). The blood perfusion defect is because of the fact that malignant lesions grow faster than benign ones, and local areas of the tumor are prone to necrosis with the increase in tumor volume, which is manifested as local blood perfusion defects during enhanced angiography (17,18). Tumor cells could destroy the basement membrane, penetrate the lower stroma, and grow outward in a crab-foot shape (13). Moreover, tumor cells tend to grow outward along existing blood vessels and integrate them as tumor expands (19). In addition to tumor angiogenesis, there are non-angiogenic mechanisms of vascular simulation such as vessel co-option and angiogenic mimicry (20,21). All of these factors may be the reasons for radial or penetrating vessels. Moreover, the same reasons explain the features of hyper-enhancement and earlier wash-in time. New tumor vessels are often formed as defective tumor endothelial cells and tumor pericytes with an irregular shape, high tortuosity, and low vasoreactivity, resulting in sluggish blood flow, which is performed as later wash-out time on CEUS (22). The bubble half-life is tens of minutes in vivo, and the time for contrast agent bubbles to start infusing breast tissue and fade varies from person to person (23). The characteristics of wash-in and wash-out time of lesions in this study were compared with those of the surrounding breast tissue to avoid the influence of contrast agent actuation duration.
CEUS combined with BI-RADS can improve the diagnostic efficiency for breast lesions. In this study, CEUS combined with BI-RADS showed a sensitivity of 84.8%, a specificity of 85.7% and an AUC value of 0.921. In the study conducted by Zhao et al. (24), CEUS combined with BI-RADS showed a sensitivity of 86.6%, a specificity of 96.8% and an AUC value of 0.917. Evaluation of blood vessels is helpful in differentiating between benign and malignant tumors. CEUS uses a 2.5-nanometer-sized microbubble and increases the contrast between microbubbles and background scattering through modern nonlinear imaging techniques so that the microcirculation in the lesion can be observed by the harmonic signal generated by the vibration of the microbubbles, and the detection ability of blood flow improved.
CEUS shows difference in diagnosis efficiency between lesions of different sizes. The overall model worked for lesions >1.0 cm (Groups 2 and 3), but was inefficient for lesions ≤1.0 cm (Group 1). It is inappropriate to use the same diagnostic model to diagnose lesions of different sizes. A previous study by Li et al. (6) reported that for lesions with >2.0 cm size, CEUS showed differences in enhancement degree, margin, homogeneity, radial or penetrating vessels and scope, with a sensitivity of 87.7%, a specificity of 99.4%, and an AUC value of 0.812. Conversely, for lesions with ≤2.0 cm size, CEUS showed differences in all parameters except for heterogeneity and had a sensitivity of 70.8%, a specificity of 92.7%, and an AUC value of 0.778. However, their study distinguished lesions only by 2.0 cm, which was not sufficiently accurate and did not solve the problem of how to improve the diagnostic efficiency of small lesions.
To explore more suitable diagnostic models for lesions of different sizes, various diagnostic models were established. In Group 1, different parameters were included in M1 to provide a new diagnostic criterion for lesions ≤1.0 cm. More features, such as earlier wash-in time and ill-defined margin, were added to M1 than M0, and blood perfusion defects and wash-out time were eliminated. As a result, the diagnostic efficiency of M1 combined with BI-RADS has improved. Wan et al. (25) found that heterogeneous enhancement and perfusion defects were detected more frequently in large tumors (size, >2.0 cm) than in small tumors (size, ≤2.0 cm). It was explained by the phenomena that the inhomogeneous distribution of the vasculature system and internal necrosis tend to appear in large tumors. Buadu et al. (26) reported that MVD and uneven distribution play a major role in heterogeneous enhancement on CEUS. In addition, the larger the tumor size, the more likely it is to have an internal hypoxic environment, uneven distribution of vessels and internal necrosis with the tumor growth. Thus, different parameters need to be considered for small lesions, which is also the reason for the poor diagnostic efficacy of previous applications of CEUS for small lesions.
In Groups 2 and 3, although new models (M2 and M3) included different factors from the original model (M0), diagnostic efficiency of M2 and M3 could not be significantly improved over that of M0. Therefore, M0 adapted perfectly for Groups 2 and 3, and there was no need to build different models to apply to breast lesions >1.0 cm.
This study has some limitations. First, this was a retrospective analysis, and only patients who underwent both US and CEUS were included, which could indicate a selection bias. Second, a quantitative analysis was not performed. Third, the influence of different pathological types on CEUS features was not evaluated. A multicenter prospective study is needed, and more data should be collected for further simplification and validation in the future.
Conclusions
In conclusion, the diagnostic performance of CEUS is closely related to lesion size. Establishing a new diagnostic model for lesions ≤1.0 cm can improve diagnostic performance of CEUS for breast lesions ≤1.0 cm.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation (grant number: 82071925).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-223/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-223/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-223/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-223/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was a retrospective clinical diagnostic test, approved by the Ethics Committee of the Chinese PLA General Hospital (No. S2021-683-01). Informed consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen C, Wang Y, Niu J, et al. Domain Knowledge Powered Deep Learning for Breast Cancer Diagnosis Based on Contrast-Enhanced Ultrasound Videos. IEEE Trans Med Imaging 2021;40:2439-51. [Crossref] [PubMed]
- Liang X, Li Z, Zhang L, et al. Application of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Different Molecular Subtypes of Breast Cancer. Ultrason Imaging 2020;42:261-70. [Crossref] [PubMed]
- Li SY, Niu RL, Wang B, et al. Determining whether the diagnostic value of B-ultrasound combined with contrast-enhanced ultrasound and shear wave elastography in breast mass-like and non-mass-like lesions differs: a diagnostic test. Gland Surg 2023;12:282-96. [Crossref] [PubMed]
- He H, Wu X, Jiang M, et al. Diagnostic accuracy of contrast-enhanced ultrasound synchronized with shear wave elastography in the differential diagnosis of benign and malignant breast lesions: a diagnostic test. Gland Surg 2023;12:54-66. [Crossref] [PubMed]
- Chen Y, Tang L, Du Z, et al. Factors influencing the performance of a diagnostic model including contrast-enhanced ultrasound in 1023 breast lesions: comparison with histopathology. Ann Transl Med 2019;7:647. [Crossref] [PubMed]
- Li C, Yao M, Shao S, et al. Diagnostic efficacy of contrast-enhanced ultrasound for breast lesions of different sizes: a comparative study with magnetic resonance imaging. Br J Radiol 2020;93:20190932. [Crossref] [PubMed]
- Lagendijk M, van Maaren MC, Saadatmand S, et al. Breast conserving therapy and mastectomy revisited: Breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer 2018;142:165-75. [Crossref] [PubMed]
- Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS® Ultrasound. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013:35-75.
- Wan C, Du J, Fang H, et al. Evaluation of breast lesions by contrast enhanced ultrasound: qualitative and quantitative analysis. Eur J Radiol 2012;81:e444-50. [Crossref] [PubMed]
- Jia C, Niu Q, Liu L, et al. Value of an expanded range of lesions on contrast-enhanced ultrasound for the diagnosis of hypervascular breast masses. Gland Surg 2023;12:824-33. [Crossref] [PubMed]
- Goubran HA, Kotb RR, Stakiw J, et al. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis 2014;7:9-18. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Welch DR, Hurst DR. Defining the Hallmarks of Metastasis. Cancer Res 2019;79:3011-27. [Crossref] [PubMed]
- Şener E, Şipal S, Gündoğdu C. Comparison of Microvessel Density with Prognostic Factors in Invasive Ductal Carcinomas of the Breast. Turk Patoloji Derg 2016;32:164-70. [Crossref] [PubMed]
- Li YJ, Wen G, Wang Y, et al. Perfusion heterogeneity in breast tumors for assessment of angiogenesis. J Ultrasound Med 2013;32:1145-55. [Crossref] [PubMed]
- Ternifi R, Wang Y, Polley EC, et al. Quantitative Biomarkers for Cancer Detection Using Contrast-Free Ultrasound High-Definition Microvessel Imaging: Fractal Dimension, Murray's Deviation, Bifurcation Angle & Spatial Vascularity Pattern. IEEE Trans Med Imaging 2021;40:3891-900. [Crossref] [PubMed]
- Metz S, Daldrup-Unk HE, Richter T, et al. Detection and quantification of breast tumor necrosis with MR imaging: value of the necrosis-avid contrast agent Gadophrin-3. Acad Radiol 2003;10:484-90. [Crossref] [PubMed]
- Uhlig J, Fischer U, Surov A, et al. Contrast-enhanced cone-beam breast-CT: Analysis of optimal acquisition time for discrimination of breast lesion malignancy. Eur J Radiol 2018;99:9-16. [Crossref] [PubMed]
- Sander LM. Modeling contact guidance and invasion by cancer cells. Cancer Res 2014;74:4588-96. [Crossref] [PubMed]
- Kuczynski EA, Vermeulen PB, Pezzella F, et al. Vessel co-option in cancer. Nat Rev Clin Oncol 2019;16:469-93. [Crossref] [PubMed]
- Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 2017;20:409-26. [Crossref] [PubMed]
- Neeman M. Perspectives: MRI of angiogenesis. J Magn Reson 2018;292:99-105. [Crossref] [PubMed]
- Albrecht T, Blomley M, Bolondi L, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med 2004;25:249-56. [Crossref] [PubMed]
- Zhao H, Xu R, Ouyang Q, et al. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol 2010;73:288-93. [Crossref] [PubMed]
- Wan CF, Du J, Fang H, et al. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology 2012;262:450-9. [Crossref] [PubMed]
- Buadu LD, Murakami J, Murayama S, et al. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology 1996;200:639-49. [Crossref] [PubMed]



