
A modified single-needle continuous suture of duct-to-mucosa pancreaticojejunostomy in pancreaticoduodenectomy
Highlight box
Key findings
• A modified single-needle continuous suture (SNCS) of duct-to-mucosa pancreaticojejunostomy (PJ) is a facile, safe, and effective PJ technique, and worthy of wide use.
What is known and what is new?
• Pancreatoenteric anastomotic failure is the main cause of pancreatic fistula after pancreaticoduodenectomy (PD). Currently, controversy persists concerning which anastomosis is best.
• This study describes in detail the modified SNCS of duct-to-mucosa PJ. It simplifies the procedure and does not increase the rate of pancreatic fistula.
What is the implication, and what should change now?
• We believe that this modified SNCS of duct-to-mucosa PJ can be used as an optional anastomotic method for PD and merits promotion and application.
Introduction
Pancreaticoduodenectomy (PD) is a complex abdominal operation that remains the only curative treatment for periampullary neoplasms (1). Although postoperative mortality has significantly fallen with improved surgical techniques and postoperative management, the incidence of postoperative complications remains high, ranging from 32% to 52%. Postoperative pancreatic fistula (POPF) is the most common and lethal complication (2). Currently, laparoscopic PD (LPD) has been widely used and it has less intraoperative bleeding and shorter hospital stays. However, no difference was noted in POPF rate and other postoperative complications (3).
Multiple factors have been linked to POPF, including individual characteristic, operation approaches, and skills (4). The pre- and post-managements, including amelioration of patients nutritional status, improvement of anastomosis technique, postoperative drainage, and somatostatin usage, have been shown to prevent the incidence of POPF (5). Several modified anastomotic methods have been proposed to obtain stable anastomosis, including end-to-side duct-to-mucosa pancreaticojejunostomy (PJ), invagination PJ, binding PJ, and pancreatogastrostomy (PG). The duct-to-mucosa PJ is the most widely used one in LPD (6), which simultaneously guarantees mucosal continuity and anastomotic patency, favoring anastomoses healing. Since the laparoscopic surgery greatly increases the difficulty of anastomosis, especially for delicated pancreatic ducts, the advanced technique is still demanding.
In this study, a modified single-needle continuous suture (SNCS) of duct-mucosa PJ was developed, which aimed to simplify anastomotic process and improve the anastomotic quality. We described a SNCS method, also compared the outcomes of patients who have received SNCS and double-layer continuous suture (DLCS) of duct-mucosa PJ in open PD (OPD). Our data demonstrated that SNCS is reliable, safe, fast, and effective anastomotic technology. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-340/rc).
Methods
Patients
Two hundred and sixty-six patients who underwent PD at Department of Pancreatic and Biliary Surgery, The First Affiliated Hospital of Harbin Medical University were involved in this study. Among them, 130 patients underwent DLCS between January 2019 and December 2020, while 136 patients underwent SNCS (73 and 63 who had OPD and LPD respectively) between January 2021 and May 2023. All the surgeries were performed by the same surgical team and the PJ were performed by the same surgeon. The inclusion criteria included a resectable benign and malignant disorders of the pancreatic head and periampullary mass. The better condition which could tolerate anesthesia and surgery. The exclusion criteria included those described by NCCN guideline.
All preoperative, perioperative, and postoperative data were collected from hospital records. Preoperative data included gender, age, body mass index (BMI), American Society of Anesthesiologists (ASA) score, comorbidities, and operation method. Perioperative data included operative time, duration of PJ, estimated blood loss (EBL), intraoperative blood transfusion, diameter of MPD, and pancreatic texture. The alternative fistula risk score (a-FRS) was calculated by following formula: P = exp[−3.136 + 0.947 (texture) + 0.0679 (BMI) − 0.385 (PD size)]/{1 + exp[−3.136 + 0.947 (texture) + 0.0679 (BMI) − 0.385 (PD size)]}, (P = probability; texture 1 = soft, 0 if not soft; PD size = pancreatic duct size in mm) according to pancreatic texture, pancreatic duct size, and BMI. Postoperative data included histopathologic diagnosis, postoperative hospital stay, postoperative complications, 90-day readmission, reoperation, and 90-day mortality. Postoperative complications included biliary leak, abdominal fluid collection, delayed gastric emptying (DGE), post-pancreatectomy hemorrhage (PPH), infection (pulmonary or urinary tract infections, wound infection), and pancreatic fistula.
POPF definition was based on the 2016 guidelines by the International Study Group of Pancreatic Fistula (ISGPF) (7). Grade A was indicated by biochemical leaks without clinical impact, while grade B and C were considered clinically relevant POPF (CR-POPF). Amylase content in the drainage fluid was measured daily for the first 3 postoperative days and afterwards where necessary. PPH and DGE were defined according to ISGPS definitions (8,9). Patients were stratified by low-risk (P<5%) intermediate-risk (P=5% to 20%) and high-risk (P>20%) by a-FRS. Timing of drainage removal was at the discretion of the surgeon. Our retrospective study was approved by the institutional Ethics Committees of the First Affiliated Hospital of Harbin Medical University (approval number: IRB-AF/SC-04/02.0) and conducted in accordance with the ethical guidelines of the Declaration of Helsinki (as revised in 2013) and individual consent for this retrospective analysis was waived.
Surgical techniques
The standard PD removed pancreatic head, duodenum, proximal jejunum, common bile duct, gall bladder, and a segment of the stomach. The child method was used for reconstructing PJ, cholangioenterostomy, and gastrojejunostomy sequentially.
SNCS-PJ procedure
After removal of the PD specimen, SNCS-PJ was performed (Figures 1,2). First, a small incision (size of the pancreatic duct) was made in the contralateral jejunal wall using electrocautery (Figure 1A). The posterior suturing layer was then sutured using a continuous-suture technique, with double needles with a 4-0 prolene suture. The needle entry site was in the midline of the pancreatic section, 1–2 mm from the superior edge of the pancreas (point A) (Figure 2A). The exit points of the needles were approximately 0.5–1 cm from the edge of the pancreatic incision in the posterior wall of the pancreas. The main pancreatic duct (MPD) and the jejunum mucous membrane were sutured when reaching pancreatic duct. Generally, three stitches were needed for the anterior and posterior half of pancreatic duct and the small hole of the jejunum. The pancreatic duct stitch included enough pancreatic parenchyma and duct and the jejunal wall stitch included the whole layer of the jejunal wall (Figures 1B,2B). A stent tube matching the size of pancreatic duct without fixation was placed in the MPD and jejunum. The stent tube was cut 2–4 side holes at the insertion end. The length of stent in the pancreatic duct was 4–5 cm, and the length in the jejunum was approximately 8–10 cm, beyond the bilioenteric anastomotic site. The same 4-0 prolene suture was used to suture the anterior suturing layer by the same protocol. The exit points of the needles were in the midline of the pancreatic section to avoid dead space (Figures 1C,2C). After the posterior and anterior wall were sutured, the suture is gently pulled taut and tied. During suturing, care should be taken to ensure that the suture does not become tangled. When tightening the sutures, slow and continuous force should be used to maintain proper tension. The needle number between point B and C should be added when the MPD is obviously delated.


DLCS-PJ procedure
After removal of the PD specimen, DLCS-PJ was performed (Figures 3,4). First, a small incision (size of the pancreatic duct) was made in the contralateral jejunal wall. Then, the posterior suturing layer was sutured using a continuous-suture technique with a 4-0 prolene suture (Figures 3A,4A). A stent tube was placed in the MPD and jejunum. The pancreatic duct-to-jejunal mucosa was continuously sutured by absorbable 5-0 suture material (Figures 3B,4B). Finally, another 4-0 prolene suture was used to suture the anterior suturing layer by the same protocol of posterior suturing layer (Figure 3C,4C).
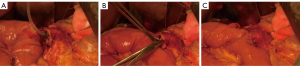
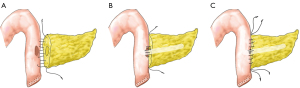
A continuous suture was taken for cholangiojejunostomy and a stapler was used for the gastrojejunostomy in both groups. After anastomosis, two prophylactic drains were placed under the pancreaticoenteric and bilioenteric anastomosis.
Statistical analysis
Propensity score matching (PSM) was performed in a 1:1 ratio to reduce bias between the SNCS-PJ and DLCS-PJ groups in OPD. Nearest neighbor matching with a calliper width of 0.02 standard deviations of the logit of the propensity score was used. Covariates were gender, age, BMI, ASA, pancreatic texture, MPD, and pathological diagnosis. Continuous data were plotted as the mean ± standard deviation (SD) or median (range), based on data distribution. Categorical variables were presented as counts and proportions. The chi-square test (or Fisher exact test) was used to compare the statistical difference and P value <0.05 was considered as statistically significant. Statistical analyses were performed by SPSS 26.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 266 patients were included for analysis. SCNS PJ was performed in 136 patients (n=73 in the OPD group and n=63 in the LPD group) and DLCS PJ in 130 patients. After PSM, 66 patients from each group were successfully matched in OPD. The pre- and post-PSM characteristics of patients are shown on Table 1. There was no difference between the two groups after PSM.
Table 1
| Variables | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| SNCS (n=73) | DLCS (n=130) | P | SNCS (n=66) | DLCS (n=66) | P | ||
| Gender | 0.036* | 0.862 | |||||
| Male | 35 (47.9) | 82 (63.1) | 35 (53.0) | 34 (52.5) | |||
| Female | 38 (52.1) | 48 (36.9) | 31 (47.0) | 32 (48.5) | |||
| Age (years) | 61 [29–76] | 61 [25–80] | 0.408 | 61 [29–76] | 61 [26–79] | 0.848 | |
| BMI (kg/m2) | 23.5 [17.4–40.3] | 22.4 [16.3–33.3] | 0.129 | 23.1 [17.4–40.3] | 23.2 [17.9–29.3] | 0.911 | |
| ASA score | 0.185 | 0.600 | |||||
| II | 30 (41.1) | 66 (50.8) | 29 (43.9) | 32 (48.5) | |||
| III | 43 (58.9) | 64 (49.2) | 37 (56.1) | 34 (51.5) | |||
| Pancreatic texture | 0.046* | 0.481 | |||||
| Soft | 27 (37.0) | 67 (51.5) | 36 (54.5) | 40 (60.6) | |||
| Hard | 46 (63.0) | 63 (48.5) | 30 (45.5) | 26 (39.4) | |||
| MPD size | 0.503 | 0.615 | |||||
| <3 mm | 38 (52.1) | 74 (56.9) | 37 (52.1) | 34 (47.9) | |||
| ≥3 mm | 35 (47.9) | 56 (43.1) | 34 (47.9) | 37 (52.1) | |||
| Diagnosis | 0.747 | 0.930 | |||||
| Pancreatic ductal adenocarcinoma | 31 (42.5) | 49 (37.7) | 29 (43.9) | 30 (45.5) | |||
| Ampullary adenocarcinoma | 5 (6.8) | 11 (8.5) | 5 (7.6) | 7 (10.6) | |||
| Distal cholangiocarcinoma | 21 (28.8) | 36 (27.7) | 17 (25.8) | 14 (21.2) | |||
| Duodenal adenoma | 9 (12.3) | 25 (19.2) | 9 (13.6) | 11 (16.7) | |||
| Pancreatic neuroendocrine tumor | 1 (1.4) | 1 (0.8) | 1 (1.5) | 1 (1.5) | |||
| Pancreatic cystic tumors | 4 (5.5) | 3 (2.3) | 3 (4.5) | 1 (1.5) | |||
| Others | 2 (2.7) | 5 (3.8) | 2 (3.0) | 2 (3.0) | |||
| Comorbidity | 0.980 | 0.600 | |||||
| None | 40 (54.8) | 71 (54.6) | 34 (51.5) | 37 (56.1) | |||
| One or more | 33 (45.2) | 59 (45.4) | 32 (48.5) | 29 (43.9) | |||
Data are presented as n (%) or median [range]. *, P<0.05. SNCS, single-needle continuous suture; DLCS, double-layer continuous suture; OPD, open pancreaticoduodenectomy; PSM, propensity score matching; BMI, body mass index; ASA, American Society of Anesthesiologists; MPD, main pancreatic duct.
The operative and postoperative details after PSM in OPD are shown in Table 2. The median operation times were shorter in the SNCS group (median 220 vs. 225 minutes, P=0.045). And the median PJ duration was significantly shorter in the SNCS group (median 9 vs. 13 minutes, P<0.001). There was no difference in CR-POPF (9.1% vs. 21.2%, P=0.052). The incidences of DGE, bile leakage, abdominal fluid collection, PPH, and infection were 13.6%, 10.6%, 10.6%, 6.1%, 19.7%, respectively. The results were similar between the two groups. The re-operation was taken for 1 patient (1.5%) in the SNCS group and 2 patients (3.0%) in DLCS group due to intra-abdominal hemorrhages related to POPF. Two patients died in the SNCS group because of intra-abdominal hemorrhage and organ failure, one patient died in DLCS group because of intra-abdominal hemorrhage. No significant difference was observed in 90-day readmission, 90-day mortality, postoperative hospital stay, and reoperation.
Table 2
| Variables | SNCS (n=66) | DLCS (n=66) | P value |
|---|---|---|---|
| Operation time (min) | 220 [170–320] | 225 [180–330] | 0.045* |
| PJ time (min) | 9 [7–15] | 13 [9–20] | <0.001* |
| EBL (mL) | 200 [50–1,000] | 200 [50–800] | 0.784 |
| Required transfusion | 8 (12.1) | 7 (10.6) | 0.573 |
| Postoperative complications | |||
| DGE | 9 (13.6) | 8 (12.1) | 0.795 |
| Grade A | 2 (3.0) | 1 (1.5) | >0.99 |
| Grade B | 3 (4.5) | 3 (4.5) | >0.99 |
| Grade C | 4 (6.1) | 4 (6.1) | >0.99 |
| Biliary fistula | 7 (10.6) | 10 (15.2) | 0.436 |
| Abdominal fluid collection | 7 (10.6) | 7 (10.6) | >0.99 |
| PPH | 4 (6.1) | 7 (10.6) | 0.345 |
| Grade A | 1 (1.5) | 3 (4.5) | 0.612 |
| Grade B | 3 (4.5) | 3 (4.5) | >0.99 |
| Grade C | 0 (0) | 1 (1.5) | >0.99 |
| Infection | 13 (19.7) | 9 (13.6) | 0.350 |
| CR-POPF | 6 (9.1) | 14 (21.2) | 0.052 |
| Grade B | 4 (6.1) | 10 (15.2) | 0.090 |
| Grade C | 2 (3.0) | 4 (6.0) | 0.676 |
| Postoperative hospital stay (days) | 18 [10–48] | 19 [10–61] | 0.199 |
| 90-day readmission | 0 (0.0) | 0 (0.0) | >0.99 |
| 90-day mortality | 2 (3.0) | 1 (1.5) | >0.99 |
| Reoperation | 1 (1.5) | 2 (3.0) | >0.99 |
Data are presented as n (%) or median [range]. *, P<0.05. PJ, pancreaticojejunostomy; SNCS, single-needle continuous suture; DLCS, double-layer continuous suture; PSM, propensity score matching; EBL, estimated blood loss; DGE, delayed gastric emptying; PPH, post-pancreatectomy hemorrhage; CR-POPF, clinically relevant postoperative pancreatic fistulas.
A feasibility of SNCS-PJ in LPD was assessed. Patient characteristics and perioperative data are summarized in Table 3. A total of 33 males and 30 females were included, with a median age of 60 (range, 18–73) years. Thirty-five patients with soft pancreas and the MPD of 36 patients was less than 3 mm. Median operative time was 290 (range, 240–470) minutes. The median duration of PJ was 17 (range, 8–30) minutes and the median EBL was 100 (range, 50–800) mL. The CR-POPF rate was 9.5%. The incidence of grade B POPF was 7.9% and grade C was 1.6%. Eight (12.7%) patients who had isolated DGE and 4 patients (6.3%) with bile leak were treated conservatively. The abdominal fluid collection occurred in 4 patients (6.3%) and infection in 4 patients (6.3%). The PPH occurred in three patients and one of them received re-operation. The median length of postoperative hospital stay was 18 (range, 9–45) days and there was no readmission and postoperative mortalities in the 90-day follow-up period.
Table 3
| Variables | Data |
|---|---|
| Gender | |
| Male | 33 (52.4) |
| Female | 30 (47.6) |
| Age (years) | 60 [18–73] |
| BMI (kg/m2) | 23.2±3.1 |
| ASA score | |
| II | 31 (49.2) |
| III | 32 (50.8) |
| Pancreatic texture | |
| Soft | 35 (55.6) |
| Hard | 28 (44.4) |
| MPD size | |
| <3 mm | 36 (57.1) |
| ≥3 mm | 27 (42.9) |
| Diagnosis | |
| Pancreatic ductal adenocarcinoma | 21 (33.3) |
| Ampullary adenocarcinoma | 6 (9.5) |
| Distal cholangiocarcinoma | 16 (25.4) |
| Duodenal adenoma | 8 (12.7) |
| Pancreatic neuroendocrine tumor | 2 (3.2) |
| Pancreatic cystic tumors | 6 (9.5) |
| Others | 4 (6.3) |
| Comorbidity | |
| None | 43 (68.3) |
| One or more | 20 (31.7) |
| Operation time (min) | 290 [240–470] |
| PJ time (min) | 17 [8–30] |
| EBL (mL) | 100 [50–800] |
| Required transfusion | 2 (3.2) |
| Postoperative complications | |
| DGE | 8 (12.7) |
| Grade A | 1 (1.6) |
| Grade B | 4 (6.3) |
| Grade C | 3 (4.8) |
| Biliary fistula | 4 (6.3) |
| Abdominal fluid collection | 4 (6.3) |
| PPH | 3 (4.8) |
| Grade A | 0 (0.0) |
| Grade B | 1 (1.6) |
| Grade C | 2 (3.2) |
| Infection | 4 (6.3) |
| CR-POPF | 6 (9.5) |
| Grade B | 5 (7.9) |
| Grade C | 1 (1.6) |
| Postoperative hospital stay (days) | 18 [9–45] |
| 90-day readmission | 0 (0.0) |
| 90-day mortality | 0 (0.0) |
| Reoperation | 1 (1.6) |
Data are presented as n (%), median [range], or mean ± SD. LPD, laparoscopic pancreaticoduodenectomy; SNCS, single-needle continuous suture; BMI body mass index; ASA, American Society of Anesthesiologists; MPD, main pancreatic duct; PJ, pancreaticojejunostomy; EBL, estimated blood loss; DGE, delayed gastric emptying; PPH, post-pancreatectomy hemorrhage; CR-POPF, clinically relevant postoperative pancreatic fistulas; SD, standard deviation.
Patients with SNCS in OPD and LPD were stratified according to the a-FRS in three groups: low-risk (47 patients, P<5%), intermediate-risk (57 patients, P=5% to 20%), and high-risk (32 patients, P>20%). There was no difference between the low-, intermediate-, and high-risk groups with respect to CR-POPF (P>0.05) (Table 4). Likewise, no significant differences in pancreatic texture, MPD size, and operation method (P>0.05) (Table 5).
Table 4
| Variables | Low (<5%) (n=47) | Intermediate (5–20%) (n=57) | High (>20%) (n=32) | P value |
|---|---|---|---|---|
| CR-POPF | 1 (2.1) | 6 (10.5) | 5 (15.6) | 0.067 |
| Grade B | 1 (2.1) | 5 (8.8) | 3 (9.4) | 0.247 |
| Grade C | 0 (0.0) | 1 (1.8) | 2 (6.3) | 0.151 |
Data are presented as n (%). CR-POPF, clinically relevant postoperative pancreatic fistulas; SNCS, single-needle continuous suture; a-FRS, alternative fistula risk score.
Table 5
| Variables | MPD size | Pancreatic texture | Operation methods | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Small MPD (<3 mm) (n=75) | Large MPD (≥3 mm) (n=61) | P value | Soft pancreas (n=63) | Hard pancreas (n=73) | P value | OPD (n=73) | LPD (n=63) | P value | |||
| CR-POPF | 9 (12.2) | 3 (4.8) | 0.134 | 8 (12.9) | 4 (5.4) | 0.125 | 6 (8.2) | 6 (9.5) | 0.789 | ||
| Grade B | 7 (9.5) | 2 (3.2) | 0.267 | 6 (9.7) | 3 (4.1) | 0.333 | 4 (5.5) | 5 (7.9) | 0.819 | ||
| Grade C | 2 (2.7) | 1 (1.6) | >0.99 | 2 (3.2) | 1 (1.4) | 0.877 | 2 (2.7) | 1 (1.6) | >0.99 | ||
Data are presented as n (%). CR-POPF, clinically relevant postoperative pancreatic fistulas; SNCS, single-needle continuous suture; MPD, main pancreatic duct; OPD, open pancreaticoduodenectomy; LPD, laparoscopic pancreaticoduodenectomy.
Discussion
The technical maturity of PD is increasing, but the incidence of POPF is still high (2). Numerous risk factors are associated with pancreatic fistula, including pancreatic factors, patient factors, and operative factors, particularly pancreatoenteric anastomotic techniques, which has been termed the “Achilles’ heel” of PD (4). Although there are various advanced PJ techniques for reducing POPF, none of them seems to be the best.
Two types of anastomoses (PG and PJ) are widely used for pancreatic-digestive tract anastomosis, PJ remains the best choice for pancreatic remnant reconstruction (7). Generally, PJ includes duct-to-mucosa anastomosis, binding anastomosis, and invagination anastomosis, none of them has clear advantage over the others. Bassi et al. (10) compared POPF incidence in patients receiving duct-to-mucosa anastomosis or invagination anastomosis and no difference was found. However, a recent meta-analysis suggested that invagination anastomosis is superior to duct-to-mucosa anastomosis with respect to POPF (11). Senda et al. (12) reached the same conclusion but in patients with soft pancreatic texture, they found that invagination anastomosis is superior to duct-to-mucosa anastomosis when dealing with a soft pancreas. Bai et al. have suggested that the incidence of CR-POPF with invagination anastomosis is significantly higher than that in duct-to-mucosa anastomosis (17.6% vs. 3.1%), with more severe complications (13). All of the differences above may be due to different definitions of POPF, surgeon experience, and variations in surgical technique. Theoretically, duct-to-mucosa anastomosis retains mucosal continuity and anastomotic patency. The pancreatic surface section is covered by jejunal serosal, which protects the pancreatic remnant from corrosion by pancreatic juice. The duct-to-mucosa has been widely used in both OPD and LPD. The SNCS follows the duct-to-mucosa anastomosis protocol. Furthermore, the number of layers in anastomosis also affect POPF incidence. Kwon et al. (14) described a single-layer anastomosis, which was considered as convenient and feasible. Several studies indicated that the single-layer technique is superior with regards to anastomotic blood supply, thereby reducing pancreatic fistula (15,16). Additionally, Lee et al. (17) showed that duct-to-mucosa anastomosis with continuous suture was safe and with low morbidity, which was favored by surgeons in LPD (18). Previous studies have suggested that even a small pinhole may cause pancreatic fistula, thus, higher number of sutures for anastomosis should be avoided. Regarding suture material, a study found that DLCS of duct-mucosa PJ, using only one polypropylene monofilament suture, did not increase the incidence of POPF (19). In comparison to absorbable suture material absorbable suture material, polypropylene monofilament suture is difficult to degrade, has controllable tension, and is easy to knot tying. Therefore, it is more applicable for single-layer continuous suture.
In recent years, novel and modified PJ techniques constantly being proposed and either of them has its own characteristics. In this study, a modified duct-mucosa anastomosis method was described and clinical outcomes was evaluated. Our approach simplified the anastomosis procedure by one continuous stitch throughout. After PSM, the rate of CR-POPF rate in the SNCS group was lower and the PJ time was shorter. Our data showed that the incidence of complications is within an acceptable range in LPD, which suggests that SNCS-PJ is equally feasible in LPD. Compared with recent reports of PJ techniques with the updated ISGPS definition (18,20-31) (Table 6), the duct-to-mucosa anastomosis is the main anastomosis method, especially in LPD. The incidence of CR-POPF is relatively low (1.2–3.2% in OPD, 0–9.1% in LPD) after duct-mucosa anastomosis, which is comparable with our results. Meanwhile, the PJ time is shorter in our study. Liu et al. (32) have described a one-layer suture PJ technique which is a little similar to ours. In their study, the SNCS was used to suture pancreatic duct to the jejunal mucosa to prevent anastomotic misplacement and serve a protective function for stent tube. They sutured the anterior suturing layer without replacing the stitches, which obtained a coherently and smoothly anastomosis process and lower incidence of POPF (6.1%). However, they were not classified by the updated ISGPS definition.
Table 6
| Source | Case No. | Methods | Time (min) | CR-POPF (%) | 90-day mortality (%) |
|---|---|---|---|---|---|
| OPD | |||||
| Zeng et al. (23), 2020 | 63 | DTM (one-layer: interrupted) | NA | 3.2 | 3.2 |
| Wang et al. (24), 2020 | 199 | IG (end-to-side: running) | NA | 14.1 | NA |
| Wu et al. (26), 2019 | 82 | DTM-PJ (out layer: running; DTM layer: interrupted) | 9.3 | 1.2 | 0 |
| Jung et al. (22), 2021 | 125 | IG-PJ (end-to-side: interrupted) | NA | 20.5 | 1.64 |
| Ferencz et al. (25), 2020 | 130 | IG-PJ (end-to-side: purse string suture) | NA | 6.9 | 0.7 |
| Zhang et al. (31), 2023 | 193 | IG (end-to-side: interrupted) | 12 | 4.7 | 0 |
| Wu et al. (28), 2023 | 529 | Non-DTM-PJ (one-layer: running) | NA | 7.9 | NA |
| Ours | 66 | DTM-PJ (one-layer: running) | 9 | 9.1 | 3.0 |
| LPD | |||||
| Du et al. (27), 2019 | 31 | DTM-PJ (out layer: running; DTM layer: purse string suture) | 30.9 | 6.5 | 0 |
| Cai et al. (18), 2019 | 238 | DTM-PJ (out layer: running; DTM layer: running) | 23 | 3.8 | 0.4 |
| Sun et al. (21), 2021 | 120 | DTM-PJ (out layer: transpancreatic; DTM layer: interrupted) | 35 | 9.1 | 0.83 |
| Tang et al. (20), 2021 | 35 | DTM-PJ (out layer: running; DTM layer: running) | 34 | 0 | 0 |
| Zhao et al. (30), 2023 | 98 | DTM-PJ (one-layer: interrupted) | 17 | 4.1 | 1.0 |
| Barberio et al. (29), 2023 | 67 | IG-PJ (end-to-end: interrupted) | 21.57 | 19.3 | 4.4 |
| Ours | 63 | DTM-PJ (one-layer: running) | 17 | 9.5 | 0 |
CR-POPF, clinically relevant postoperative pancreatic fistulas; OPD, open pancreaticoduodenectomy; DTM, duct-to-mucosa; NA, not available; IG, invagination; PJ, pancreaticojejunostomy; LPD, laparoscopic pancreaticoduodenectomy.
The impact of pancreatic texture, MPD size, operation method, and a-FRS to POPF were analyzed. Our results indicated that the incidence of CR-POPF was slightly higher in patients with soft pancreas (12.9% vs. 5.4%), small MPD size (12.2% vs. 4.8%) and OPD (8.2% vs. 9.5%) without any difference (P>0.05) (Table 5). Furthermore, no difference was found among the low, intermediate and high-risk groups (P>0.05). The POPF rate in patients with soft and small MPD pancreas was favorably with those from other reports by the updated ISGPS definition. A meta-analysis (33) showed the CR-POPF rate in patients with soft pancreas is 26.2%, which is higher than our study (14.3%). Jin et al. (34) analyzed the CR-POPF rate in two-layer duct-to-mucosa anastomosis and the rates in soft pancreas and small MPD group were 27.8% and 19.2%. Several studies (35,36) have validated the a-FRS model which is useful and reliable. The CR-POPF rates in low, intermediate and high risks were relatively lower than previous reports (2.1% vs. 8.5%, 5.88%, 10.5% vs. 20.8%, 24.38% and 15.6% vs. 27.5%, 57.69%). These results indicate that the new technology is feasible, reliable, and safe, regardless of pancreatic texture, MPD size, operation method, and a-FRS stratification.
This technique has the following strengths. First, anastomosis of the pancreatic duct to the jejunal mucosa aids anastomotic healing, and avoids pancreatic stump immersion into the intestinal lumen, which may cause infection, hemorrhage, and necrosis. Second, single-layered suture anastomosis prevents POPF caused by very dense suture and suture line tearing of pancreatic tissue. Third, anterior and posterior layer sutures intersect at the midline of the pancreas, which prevents dead space and avoids high tension on the anastomosis, due to digestate accumulation in the gap. Fourth, continuous-suture anastomosis with same suture distributes tension evenly and reduces the duration of operation. Finally, this technique decreases the number of suturing and knot tying to facilitate performed under laparoscopy. In summary, the SNCS is easy to learn and launch, making it convenient for laparoscopy and reducing anastomosis time.
This study has some limitations. First, this is a retrospective study and selection bias may be unavoidable. Second, the sample size in this study is small. Third, it is a single-center study and all the operations were completed by the same surgical group. Fourth, it is evaluated short-term postoperative outcomes with a lack of long-term follow-up. Thus, a multicenter prospective randomized controlled trial is required to evaluate its technical feasibility, safety, efficacy, and long-term outcomes.
Conclusions
We introduce a modified anastomosis technique for PJ. The present study shows that the SNCS is reliable, safe and fast, and it does not increase POPF incidence regardless of pancreatic texture, MPD size, operation method, and a-FRS stratification.
Acknowledgments
We acknowledge the support of colleagues from the Department of Pancreatic and Biliary Surgery of The First Affiliated Hospital of Harbin Medical University.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-340/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-340/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-340/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-340/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki (as revised in 2013). Our retrospective study was approved by the institutional Ethics Committees of the First Affiliated Hospital of Harbin Medical University (approval number: IRB-AF/SC-04/02.0) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10-5. [Crossref] [PubMed]
- Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, et al. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery 2012;152:S56-63. [Crossref] [PubMed]
- Ausania F, Landi F, Martínez-Pérez A, et al. A meta-analysis of randomized controlled trials comparing laparoscopic vs open pancreaticoduodenectomy. HPB (Oxford) 2019;21:1613-20. [Crossref] [PubMed]
- de Castro SM, Busch OR, van Gulik TM, et al. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg 2005;92:1117-23.
- Søreide K, Labori KJ. Risk factors and preventive strategies for post-operative pancreatic fistula after pancreatic surgery: a comprehensive review. Scand J Gastroenterol 2016;51:1147-54. [Crossref] [PubMed]
- Varco RL. A method of implanting the pancreatic duct into the jejunum in the Whipple operation for carcinoma of the pancreas; case report. Surgery 1945;18:569-73.
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Bassi C, Falconi M, Molinari E, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery 2003;134:766-71. [Crossref] [PubMed]
- Hua J, He Z, Qian D, et al. Duct-to-Mucosa Versus Invagination Pancreaticojejunostomy Following Pancreaticoduodenectomy: a Systematic Review and Meta-Analysis. J Gastrointest Surg 2015;19:1900-9. [Crossref] [PubMed]
- Senda Y, Shimizu Y, Natsume S, et al. Randomized clinical trial of duct-to-mucosa versus invagination pancreaticojejunostomy after pancreatoduodenectomy. Br J Surg 2018;105:48-57. [Crossref] [PubMed]
- Bai X, Zhang Q, Gao S, et al. Duct-to-Mucosa vs Invagination for Pancreaticojejunostomy after Pancreaticoduodenectomy: A Prospective, Randomized Controlled Trial from a Single Surgeon. J Am Coll Surg 2016;222:10-8. [Crossref] [PubMed]
- Kwon YJ, Ahn BK, Park HK, et al. One layer end-to-side pancreaticojejunostomy using reinforcing suture on the pancreatic stump. Hepatogastroenterology 2013;60:1488-91. [Crossref] [PubMed]
- Wei J, Liu X, Wu J, et al. Modified One-layer Duct-to-mucosa Pancreaticojejunostomy Reduces Pancreatic Fistula After Pancreaticoduodenectomy. Int Surg 2015; Epub ahead of print. [Crossref]
- Pan SB, Geng W, Zhou DC, et al. One-layer versus two-layer duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: study protocol for a randomized controlled trial. Trials 2016;17:407. [Crossref] [PubMed]
- Lee SE, Yang SH, Jang JY, et al. Pancreatic fistula after pancreaticoduodenectomy: a comparison between the two pancreaticojejunostomy methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer: interrupted vs continuous stitches. World J Gastroenterol 2007;13:5351-6. [Crossref] [PubMed]
- Cai Y, Luo H, Li Y, et al. A novel technique of pancreaticojejunostomy for laparoscopic pancreaticoduodenectomy. Surg Endosc 2019;33:1572-7. [Crossref] [PubMed]
- Chen Y, Tan C, Zhang H, et al. Novel entirely continuous running suture of two-layer pancreaticojejunostomy using only one polypropylene monofilament suture. J Am Coll Surg 2013;216:e17-21. [Crossref] [PubMed]
- Tang W, Qiu JG, Li GZ, et al. Clinical application of "Double R" anastomosis technique in laparoscopic pancreaticoduodenectomy procedure. Medicine (Baltimore) 2021;100:e26204. [Crossref] [PubMed]
- Sun PJ, Yu YH, Li JW, et al. A Novel Anastomosis Technique for Laparoscopic Pancreaticoduodenectomy: Case Series of Our Center's Experience. Front Surg 2021;8:583671. [Crossref] [PubMed]
- Jung YK, Choi D, Lee KG. One-layer pancreaticojejunostomy using reinforcing sutures in pancreaticoduodenectomy: A single surgeon's experience with 122 cases. Asian J Surg 2021;44:286-91. [Crossref] [PubMed]
- Zeng ZL, Sun Y, Xue D, et al. Effect of six-stitch pancreaticojejunostomy on pancreatic fistula: A propensity score-matched comparative cohort study. Hepatobiliary Pancreat Dis Int 2020;19:277-83. [Crossref] [PubMed]
- Wang D, Liu X, Wu H, et al. Clinical evaluation of modified invaginated pancreaticojejunostomy for pancreaticoduodenectomy. World J Surg Oncol 2020;18:75. [Crossref] [PubMed]
- Ferencz S, Bíró Z, Vereczkei A, et al. Innovations in pancreatic anastomosis technique during pancreatoduodenectomies. Langenbecks Arch Surg 2020;405:1039-44. [Crossref] [PubMed]
- Wu T, Guo Y, Bi J, et al. Modified duct-to-mucosa versus conventional pancreaticoenterostomy for pancreaticoduodenectomy: a retrospective cohort study based on propensity score matching analysis. World J Surg Oncol 2019;17:5. [Crossref] [PubMed]
- Du Y, Wang J, Li Y, et al. Clinical application of a modified pancreatojejunostomy technique for laparoscopic pancreaticoduodenectomy. HPB (Oxford) 2019;21:1336-43. [Crossref] [PubMed]
- Wu JM, Lin YJ, Wu CH, et al. Novel Non-duct-to-Mucosa Pancreaticojejunostomy Reconstruction After Pancreaticoduodenectomy: Focus on the Occurrence of Post-pancreatectomy Hemorrhage and Intra-abdominal Abscess. Ann Surg Oncol 2023;30:5063-70. [Crossref] [PubMed]
- Barberio M, Milizia A, Pizzicannella M, et al. End-to-end invaginated pancreaticojejunostomy during minimally invasive pancreatoduodenectomy: technical description and single center experience. Surg Endosc 2023;37:7370-5. [Crossref] [PubMed]
- Zhao A, Zhu Q, Qin X, et al. A duct-to-mucosa pancreaticojejunostomy for small main pancreatic duct and soft pancreas in minimally invasive pancreaticoduodenectomy. Surg Endosc 2023;37:3567-79. [Crossref] [PubMed]
- Zhang L, Zhu X, Zhu Y, et al. Chen's penetrating-suture technique for pancreaticojejunostomy following pancreaticoduodenectomy. BMC Surg 2023;23:146. [Crossref] [PubMed]
- Liu CZ, Zhu JK, Xu Q, et al. Application of pancreaticojejunostomy with one-layer suture in pancreaticoduodenectomy: A retrospective cohort study. Int J Surg 2018;56:68-72. [Crossref] [PubMed]
- Eshmuminov D, Schneider MA, Tschuor C, et al. Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 International Study Group Pancreatic Fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB (Oxford) 2018;20:992-1003. [Crossref] [PubMed]
- Jin KM, Liu W, Wang K, et al. The individualized selection of Pancreaticoenteric anastomosis in Pancreaticoduodenectomy. BMC Surg 2020;20:140. [Crossref] [PubMed]
- Lao M, Zhang X, Guo C, et al. External validation of alternative fistula risk score (a-FRS) for predicting pancreatic fistula after pancreatoduodenectomy. HPB (Oxford) 2020;22:58-66. [Crossref] [PubMed]
- Shinde RS, Acharya R, Chaudhari VA, et al. External validation and comparison of the original, alternative and updated-alternative fistula risk scores for the prediction of postoperative pancreatic fistula after pancreatoduodenectomy. Pancreatology 2020;20:751-6. [Crossref] [PubMed]


