Safety of energy based devices for hemostasis in thyroid surgery
Introduction
Thyroid operations are increasingly performed (1,2). The Italian board of health reported 37,647 discharges for thyroid and 2,317 discharges for parathyroid surgery in 2013 (1). About 150,000 patients in the United States undergo thyroidectomy per year for benign or malignant disease (2).
In general, the essential objectives for thyroidectomy are: sparing the parathyroid glands, avoidance of injury to the laryngeal nerves, an accurate hemostasis and an excellent cosmesis (3-5).
In the last 10 years major improvements and new technologies have been proposed and applied in thyroid surgery; among these mini-invasive thyroidectomy, new devices for achieving hemostasis and dissection, intraoperative neuromonitoring, and PTH assay technology (3-5).
Energy based devices (EBD) have been developed, implemented and increasingly applied in thyroid surgery because they can provide a combined dissection and haemostatic effect (6,7). In particular, advantages of EBD have been described in terms of efficacious haemostasis, reduction of procedure-associated time, reduced incision length, less operative blood loss and transfusion need, decreased postoperative drain, pain and hospital stay (6-10). The use of EBDs has replaced hand-tying methods in many Institutions. What is more, EBD have shown similar morbidity in comparison to conventional techniques. On the contrary, a potential drawback is, for some authors, the increased health care costs (10-16). In addition, EBD are essential for endoscopic procedures (16-22).
Recurrent laryngeal nerve (RLN) palsy is the most common and serious complication of thyroid surgery. Following traction injury, thermal injury is the second most common mechanism of RLN damage during thyroidectomy (19). Intraoperative RLN thermal injuries are believed to be mostly related to the EBDs tip thermal effect, which is used for dissection or bleeding control near the RLN (19). Since EBDs are widely used for haemostasis and dissection, their safety must be assessed and the standardization of their use must be studied (19).
This paper reviews relevant medical literature published on the influence of new devices for achieving hemostasis and dissection.
Experimental study protocols on EBD safety around the RLN
EBD use proximal to the RLN presents risks related to lateral thermal spread and associated nerve damage. Different surveys describe the safety of EBD around the RLN (22-27). Such investigations are needful as they highlighted areas for correction in the adoption of the technology in thyroid and parathyroid surgery (19).
Notwithstanding, these unique reports, the experimental model proposed imposes some brief considerations about its validity.
An experimental study protocol on an EBD for thyroid surgery should include two phases.
First phase may include a “close study”, that is to investigate the safety distance of EBD application near the RLN. Evaluation of the distance range of EBD application from RLN set at standardized fashion for example 5, 4, 3, 2 and 1 mm distance.
The second phase of the protocol should include the “length time study”. Animals are included for (I) evaluation of safety duration of using EBD near the RLN; and (II) safety to dissect the RLN with EBD immediately after its application. This can be achieved, for example, after the EBD application on muscle the instrument touch the RLN 1, 2, 3, 4, 5 seconds after its activation, or immediately after activation, or immediately after activation and touching/cooling with a surrounding tissue.
The above two phases are important to try to extend into the experimental model what actually happens in clinical practice during thyroid surgery.
Additionally, different RLN injuries types were experimentally induced in a porcine model to compare morphological change (28). A porcine model of traction lesion showed only distorted outer nerve structure, whereas the thermal lesion by EBD showed severe damage in the inner endoneurium (28).
The Harmonic Focus (HF) (Ethicon, Johnson and Johnson, Cincinnati, OH, USA)
The HF is one of the most popular energy-based devices. Recent studies were to provide RLN functional data that define the safety parameters of the HF during thyroidectomy (22,24). Most these studies were prospective porcine model using continuous electrophysiologic monitoring. Piglets were used (22,24). At varying distances from the RLN, the HF was activated (activation study). The HF was also applied directly on the RLN after activation on sternocleidomastoid muscle for 10 seconds with different cooling times (cooling study). In the activation study, there was no adverse electromyography (EMG) event at more than 1 mm distance (Figure 1). In the cooling study, there was no adverse EMG event after a 10-second cooling period (22,24). When the HF was cooled on the sternocleidomastoid muscle, there was no adverse EMG event after 2 seconds cooling time. Authors concluded that, the safe distance of the HF was 1 mm, and it should be cooled for more than 10 or 2 seconds after cooling on muscle. The HF should be used in a standardized manner to avoid RLN injury (22,24).
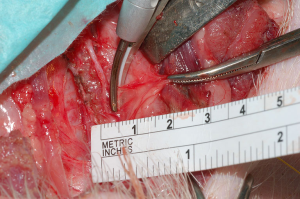
The Thunderbeat (TB) (Olympus, Japan)
TB is one of the most widely used EBDs. One study aimed to test the safety of TB during thyroidectomy (27). Four piglets weighing 30–40 kg experienced thyroidectomy while continuous intraoperative neural monitoring (CIONM) occurred, using an EMG endotracheal tube and NIM 3.0 response system. TB was applied at various distances from the RLN, and Authors assessed the safety of the protocols. Adverse EMG events did not occur at distances >3 mm from the RLN. Amplitude decreased at 2 mm from the RLN after 8 s. However, immediate loss of signal occurred at 1 mm from the RLN, likely due to immediate shrinkage of surrounding tissue after TB application. The Authors concluded that TB can be used safely at 3 mm from the RLN but must be used for <8 s at more proximal locations. This was the first report assessing the safety of TB, and findings indicate that TB should be used at least 1 mm from the RLN to avoid injury.
LigaSureTM Small Jaw (LSJ) (Medtronic, Covidien product, Mineapolis, MN, USA)
We investigated recently the electromyographic signal pattern durung the use of LSJ around the RLN and to establish a standardized technique to prevent potential nerve injuries. No previous study investigated the EMG signal pattern during the use of the LSJ around the RLN in endocrine surgery. About 14 piglets (28 RLNs) underwent C-IONM. Dynamic EMG tracings were recorded during various applications of LSJ around RLN. The protocol included: (I) close study (12 RLNs): performed in order to assess the safety distance of the use of LSJ near the RLN; (II) length time study (16 RLNs): (i) evaluation of safety duration of using LSJ near the RLN (16 RLNs); (ii) safety to dissect the RLN with LSJ immediately after its application. EMG signal remained stable at 5–2 mm distance test (totally 72 tests) (Figure 2). Using LSJ <1 mm distance determined immediate complete EMG signal loss (3RLNs) and a partial reduction of the amplitude between 50–87% (9 RLNs). The EMG signal remained stable if a lag time was at least 2 seconds (totally 96 tests). Touching the RLN immediately after LSJ activation determined EMG loss (3 RLNs) and amplitude reduction 48–70% (4 RLNs). The EMG signal remained stable if LSJ touched some surrounding tissue before reaching the other 8 RLNs (touch maneuver, totally 24 tests). Latency changes were less consistent with several signal artifacts. Injured RLN EMG did not gained recovery after 25 to 60 minutes. Monitoring confirmed type-I segmental lesion in all injured RLN. We can conclude that LSJ cause RLN injuries if a standardized technique is not used. Notably, RLN should be well-visualized before the LSJ activation. Moreover, a lag time of at least 2 seconds or touch maneuver is needed before using LSJ for dissection around the RLN. What is more, a safety margin for LSJ is set at least 2 mm.
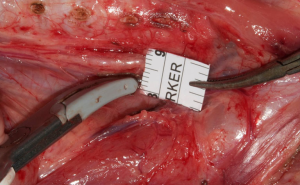
The severity of thermal injury by EBD in the clinical practice
Few studies in the literature have reported recovery data for different types of RLN injuries in the clinical practice. One study, first, attempts to classify RLN injuries and rank them by severity (28). The study was a prospective clinical study that analyzed 281 RLN injuries in which a true loss of signal was identified by IONM, and VCP was confirmed by a postoperative laryngoscope.
For each injury type, the prevalence of VCP, the time of VCP recovery, and physical changes on nerves were analyzed.
The overall VCP rate in at-risk patients/nerves was 8.9%/4.6%, respectively (28).
RLN thermal injury was quite frequent (28). The distribution of RLN injuries types, in order of frequency, was traction (71%), thermal from EBD (17%), compression (4.2%), clamping (3.4%), ligature entrapment (1.6%), suction (1.4%), and nerve transection (1.4%).
Different RLN injuries induce different morphological alterations and have different recovery outcomes. Complete recovery from VCP was documented in 91% of RLN injuries (28). The severity of thermal injuries by EBD was worst than traction injury (28). De facto, recovery time was significantly faster in the traction group compared to the other groups (P<0.001). The rates of temporary and permanent VCP were 98.6% and 1.4% for traction lesion, 72% and 28% for thermal injury by EBD, 100% and 0% for compression injury, 50% and 50% for clamping injury, 100% and 0% for ligature entrapment, 100% and 0% for suction injury, and 0% and 100% for nerve transection, respectively (28).
Conclusions
Considering the wide availability of EBD more nerve injuries caused by thermal spread are expected in the near future if a strict standardized use is not applied. EBDs are unsafe near the RLN. It is important not to directly touch the nerve with EBS immediately after the latter has been used. RLN must be well visualized before the activation. According to published results the safe margin is >1 mm. The activation time for close study is 2–4 mm, for length study is 5 seconds on muscle for most of EBDs. Experimental histological studies of vessel sealed with EBD demonstrated 1.5 to 3.3 mm thermal spread, beyond the tissue within the forceps’ jaws (22-27). Authors suggested not using the device closer than 2–3 mm to the RLN (22-27). After activation, a lag time at least 2 seconds or touching tissue is needed before the use of LSJ for blunt dissection around the RLN. In order to reduce the temperature of the tip of the LSJ the touch maneuver is useful.
However, there are limitations in all present porcine studies (22-27). Firstly, these are animal models with median sample size, and caution must be taken in translating these data into clinical context. RLN physiopathology in porcine may be different from the human, because porcine anatomy may have different tissue composition. Nevertheless, these models have been previously proven as a useful and reliable model to investigate the laryngeal EMG changes during IONM, and there was a certain level of reproducibility in each datum. Therefore, the results show a consistent tendency rather than random phenomena in piglets. Other significant drawbacks are the absence of long follow-up, postoperative laryngoscopy and in some studies the absence of a histological analysis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Salute MD. Rapporto annuale sull’attività di ricovero ospedaliero. Dati SDO 2013-2014.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Dionigi G, Bacuzzi A, Bertocchi V, et al. Safe incorporation of new technologies in thyroid surgery. Expert Rev Med Devices 2008;5:747-58. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Thyroid surgery: new approach to dissection and hemostasis. Surg Technol Int 2006;15:75-80. [PubMed]
- Dionigi G, Bacuzzi A, Boni L, et al. Influence of new technologies on thyroid surgery: state of the art. Expert Rev Med Devices 2005;2:547-57. [Crossref] [PubMed]
- Yao HS, Wang Q, Wang WJ, et al. Prospective clinical trials of thyroidectomy with LigaSure vs conventional vessel ligation: a systematic review and meta-analysis. Arch Surg 2009;144:1167-74. [Crossref] [PubMed]
- Contin P, Gooßen K, Grummich K, et al. ENERgized vessel sealing systems versus CONventional hemostasis techniques in thyroid surgery--the ENERCON systematic review and network meta-analysis. Langenbecks Arch Surg 2013;398:1039-56. [Crossref] [PubMed]
- Chang LY, O'Neill C, Suliburk J, et al. Sutureless total thyroidectomy: a safe and cost-effective alternative. ANZ J Surg 2011;81:510-4. [Crossref] [PubMed]
- Zarebczan B, Mohanty D, Chen H. A Comparison of the LigaSure and harmonic scalpel in thyroid surgery: a single institution review. Ann Surg Oncol 2011;18:214-8. [Crossref] [PubMed]
- Lang BH, Yih PC, Hung GK. Does using an energized device in open thyroidectomy reduce complications? J Surg Res 2013;181:e23-9. [Crossref] [PubMed]
- Hirunwiwatkul P, Tungkavivachagul S. A multicenter, randomized, controlled clinical trial of LigaSure small jaw vessel sealing system versus conventional technique in thyroidectomy. Eur Arch Otorhinolaryngol 2013;270:2109-14. [Crossref] [PubMed]
- Duan YF, Xue W, Zhu F, et al. FOCUS harmonic scalpel compared to conventional hemostasis in open total thyroidectomy - a prospective randomized study. J Otolaryngol Head Neck Surg 2013;42:62. [Crossref] [PubMed]
- Dionigi G, Boni L, Rausei S, et al. The safety of energy-based devices in open thyroidectomy: a prospective, randomised study comparing the LigaSure™ (LF1212) and the Harmonic® FOCUS. Langenbecks Arch Surg 2012;397:817-23. [Crossref] [PubMed]
- Dionigi G, Van Slycke S, Rausei S, et al. Parathyroid function after open thyroidectomy: A prospective randomized study for ligasure precise versus harmonic FOCUS. Head Neck 2013;35:562-7. [Crossref] [PubMed]
- Rahbari R, Mathur A, Kitano M, et al. Prospective randomized trial of ligasure versus harmonic hemostasis technique in thyroidectomy. Ann Surg Oncol 2011;18:1023-7. [Crossref] [PubMed]
- Lang BH, Ng SH, Lau LL, et al. A systematic review and meta-analysis comparing the efficacy and surgical outcomes of total thyroidectomy between harmonic scalpel versus ligasure. Ann Surg Oncol 2013;20:1918-26. [Crossref] [PubMed]
- Kwak HY, Chae BJ, Park YG, et al. Comparison of surgical outcomes between papillary thyroid cancer patients treated with the Harmonic ACE scalpel and LigaSure Precise instrument during conventional thyroidectomy: a single-blind prospective randomized controlled trial. J Surg Res 2014;187:484-9. [Crossref] [PubMed]
- Ecker T, Carvalho AL, Choe JH, et al. Hemostasis in thyroid surgery: harmonic scalpel versus other techniques--a meta-analysis. Otolaryngol Head Neck Surg 2010;143:17-25. [Crossref] [PubMed]
- Dionigi G. Energy based devices and recurrent laryngeal nerve injury: the need for safer instruments. Langenbecks Arch Surg 2009;394:579-80; author reply 581-6. [Crossref] [PubMed]
- Garas G, Okabayashi K, Ashrafian H, et al. Which hemostatic device in thyroid surgery? A network meta-analysis of surgical technologies. Thyroid 2013;23:1138-50. [Crossref] [PubMed]
- Barczyński M, Konturek A, Cichoń S. Minimally invasive video-assisted thyreoidectomy (MIVAT) with and without use of harmonic scalpel--a randomized study. Langenbecks Arch Surg 2008;393:647-54. [Crossref] [PubMed]
- Dequanter D, Lammens M, Nagy N, et al. Thyroid surgery with a harmonic scalpel: an experimental study. Med Devices (Auckl) 2016;9:139-42. [Crossref] [PubMed]
- Puram SV, Chow H, Wu CW, et al. Vocal cord paralysis predicted by neural monitoring electrophysiologic changes with recurrent laryngeal nerve compressive neuropraxic injury in a canine model. Head Neck 2016;38 Suppl 1:E1341-50. [Crossref] [PubMed]
- Wu CW, Chai YJ, Dionigi G, et al. Recurrent laryngeal nerve safety parameters of the Harmonic Focus during thyroid surgery: Porcine model using continuous monitoring. Laryngoscope 2015;125:2838-45. [Crossref] [PubMed]
- Lin YC, Dionigi G, Randolph GW, et al. Electrophysiologic monitoring correlates of recurrent laryngeal nerve heat thermal injury in a porcine model. Laryngoscope 2015;125:E283-90. [Crossref] [PubMed]
- Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery 2014;155:329-39. [Crossref] [PubMed]
- Kwak HY, Dionigi G, Kim D, et al. Thermal injury of the recurrent laryngeal nerve by THUNDERBEAT during thyroid surgery: findings from continuous intraoperative neuromonitoring in a porcine model. J Surg Res 2016;200:177-82. [Crossref] [PubMed]
- Dionigi G, Wu CW, Kim HY, et al. Severity of Recurrent Laryngeal Nerve Injuries in Thyroid Surgery. World J Surg 2016;40:1373-81. [Crossref] [PubMed]


