Intermittent neural monitoring of the recurrent laryngeal nerve in surgery for recurrent goiter
Background
Reoperative thyroid surgery is relatively rare, but still represents a major challenge, even for skilled surgeons, because of the higher incidence of complications than in primary thyroid operations, in particular hypoparathyroidism and injury to the recurrent laryngeal nerve (RLN). The RLN is at high risk during reoperations, due to the likelihood of displacement and scarring following previous neck surgery. The prevalence of RLN injury during reoperative thyroid surgery is as high as 12.5% for transient injury and up to 3.8% for permanent injury, which is a serious complication influencing the quality of life and having a wide range of symptoms (1-15) (Table 1).

Full table
The indications for reoperative surgery for thyroid disease vary, and the patients for this type of procedure are not a homogenous group (11,16-19). It is sometimes indicated for nodular recurrence after partial surgery for initially benign thyroid disease, mainly after bilateral subtotal thyroidectomy, or to complete total thyroidectomy when thyroid cancer is recognized in histopathological report after the thyroid gland is partially removed in the initial operation. Secondary thyroidectomies also include the completion thyroidectomy following a lobectomy for differentiated thyroid carcinoma, or for contralateral recurrent goiter after a previous hemithyroidectomy. In this kind of redo surgery it is generally easier for the surgeon to remove all the remaining tissue than after subtotal thyroid resection, which can leave behind extensive local scarring. Nowadays reoperative thyroid surgery is an uncommon procedure, because of the growing acceptance of total thyroid operations for benign multinodular goiter (BMNG) in the last two decades (20-22). This trend is in accordance with recommendations from the British and American Thyroid Associations, which suggest avoiding subtotal procedures for BMNG in favour of a hemi- or total thyroidectomy during the initial procedure (23).
Routine identification of the RLN has been the gold standard in thyroid surgery for three decades, and is the best way to prevent inadvertent injury and to minimize the rate of postoperative vocal cord palsy (24-28). In recent years intraoperative neuromonitoring (IONM) has gained more acceptances among endocrine surgeons and laryngologists as an adjunct to visual RLN identification (29-32). IONM of the RLN and the external branch of the superior laryngeal nerve (EBSLN) have been standardized and seem to be beneficial in high risk thyroid operations, particularly in reoperations (25,26). In recent years there have been many publications concerning the introduction of IONM, the learning curve and the technical aspects of new the technology, and assessing the prevalence of complications when utilizing IONM (17,20,25-27,29-32). But very little data concerning the incidence of RLN injury in reoperations with IONM has been published, and only a few publications have compared the RLN injury rate during secondary thyroid surgery using IONM with procedures using only visual RLN identification (16-19,33,34).
The aim of this study was to evaluate the use of intermittent IONM of the RLN in surgery for recurrent goiter. We focused not only on the final effect of IONM in reoperations, but also on practical aspects such as RLN identification in a scarred operative field and surgical strategy in reoperations.
IONM in recurrent goiter
In 1938, Lahey showed that careful dissection of the RLN does not increase the rate of RLN injury during thyroid operations; instead, it significantly reduces the frequency of RLN palsy (35). This study launched a new approach to thyroid surgery. In the following years many publications confirmed those findings (20,24,36,37). The largest study was conducted by Jatzko et al., involving 12,211 thyroid operations, and it confirmed that the prevalence of transient and permanent paresis in operations where the RLN was not visualized (7.9% and 5.2%, respectively) was significantly higher than in operations in which the nerve was visualized (2.7% and 1.2%, respectively) (24). For many years the RLN was visualized using various different techniques, including the use of magnifying glasses (21,23,24,36,37). Since Shedd’s introduction of IONM in thyroid surgery in 1966, identification of the RLN has been much easier, which has reduced postoperative complications (38). In the first randomized prospective study of 1,000 patients undergoing thyroid reoperation for recurrent goiter, Barczyński et al. showed that the prevalence of transient RLN palsy was significantly lower among patients whose surgery utilized IONM (2.9% lower in high risk patients and 0.9% lower in low-risk patients) than among those whose operations involved only RLN visualization (39). Reoperations are undoubtedly more dangerous for the RLN than primary operations (11,12,14,15), hence the utilization of IONM should be always recommended to minimize the risk of nerve injury due to certain advantages the technique has over visual identification alone.
RLN identification and mapping
The rate of RLN identification with IONM is near 100%, because neuromonitoring can locate the RLN before visual confirmation (40,41). The nerve is mapped out in the paratracheal region through probe stimulation and then visually identified through directed dissection based on the neural mapping (25). This technique is particularly beneficial in reoperations where there is scar tissue from the first thyroid operation. Barczyński et al. showed that almost 20% of the RLNs were identified with IONM before visual exposition of the nerve, and almost twice as many ramified nerves were identified in reoperations with IONM than without monitoring (P<0.001) (17). Moreover, the use of IONM significantly (P=0.02) improved identification of non-recurrent laryngeal nerves (non-RLNs) than in operations without monitoring (17).
In a scarred operating field there can be problems with hemostasis because of the high degree of vascularization. In these cases, mapping the RLN through the overlying tissue can minimize bleeding during reoperations (11,12,14). IONM is helpful in distinguishing branches of the RLN from the vessels and scarred tissue (42,43). When the nerve is imbedded in a scar, IONM should be used first for mapping and identification and then for monitoring during dissection (44-47).
Mapping the RLN is especially helpful in reoperations where the nerve is distorted in any direction, which can disorient surgeons and could lead to inadvertent injury if the nerve is mistaken for other structures (non-anatomical courses of the nerve are described later in the text). When mapping the RLN in a scarred field, 2 mA stimulation is recommended. For confirmation of the RLN or further intraoperative monitoring we use 1 mA. In cases where two similar structures are close together, lower stimulations (for example 0.5 mA) can be used to distinguish them (47,48).
The RLN in reoperative thyroid surgery
The RLN is prone to injury during thyroid surgery because it has a wide range of anatomical variants, and may be in various positions relative to the inferior thyroid artery. In about 50% of cases the nerve branches, and in 0.03% the non-RLN could be present (16-19,33,34,49). Other risk factors involved in RLN injury are hyperthyroidism, the extent of the surgical treatment and of course the surgeon’s experience with endocrine surgery (11,14). However, reoperations seem to be the major risk factor for nerve injury. As we have already stated, in thyroid reoperations the RLN may be displaced in any direction (44-47). Usually we find the nerve adherent to the lateral capsule of the remnant thyroid mass or adherent strictly to the inferior part of a recurrent goiter. Another obstacle in reoperations is (as noted earlier) massive scarring after the first operation, in which the nerve could be imbedded (16-19,33,34). A scarred operating field, in particular after subtotal thyroid operations, makes it difficult not only to find the RLN but also to separate it from other structures.
The RLN adherent to the lateral capsule of a goiter in reoperation
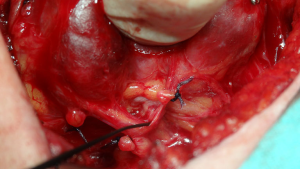
RLNs adherent to the lateral capsule of a goiter are most frequently seen in huge retrosternal recurrent goiters, many years after the first partially removed goiter (Figure 1). The nerve is dislocated from its typical course due to the remnant thyroid tissue growing close to it. There is a high risk of RLN dislocation when Zuckerkandl’s tubercle was missed in the first thyroid operation (50). In initial thyroidectomies the RLN always runs under Zuckerkandl’s tubercle, but in reoperations the RLN could be adherent to the lateral capsule of this tubercle. This leaves the nerve at great risk of inadvertent injury, because it is usually is almost invisible, in particular at the beginning of the operation. In such a position, the nerve may be mistaken for other structures, such as fibrosis or blood vessels, and transected inadvertently (33,44,45,50). Clamping or transecting any structure before careful preparation of the thyroid tissue and identification of the nerve should be avoided (25,33,47). Visual identification of the RLN in cases where the nerve is imbedded in the capsule of a goiter is very difficult and sometimes even skilled surgeons have difficulty in finding it. IONM, in particular mapping the nerve at the beginning of the reoperation, is recommended. By using IONM not only are we able to identify the nerve, but we can also separate the nerve from the goiter (46). When the nerve is dissected completely from the remaining thyroid tissue, the functional integrity of the nerve should be confirmed with IONM in accordance with the IONM Study Group recommendations (25). This final confirmation of RLN functionality is crucial in cases where the RLN runs over a huge goiter, because the nerve is particularly prone to overstretching with potential loss of function after dissection.
A potential non-anatomical RLN course like this shows that the lateral approach seems to be the most useful technique in repeat thyroid operations, since it allows the surgeons to reach the remnant tissue through a previously undisturbed plane (25).
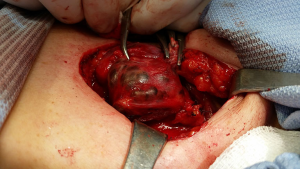
The RLN attached to the inferior part of a goiter in reoperation
This non-typical course of the nerve is seldom seem in primary thyroid operations, but should be always considered in retrosternal or mediastinal enlargements in reoperations. A huge goiter could also be involved in this type of dislocation (14,44,51). The remnant retrosternal thyroid mass, often missed during the first procedure, subsequently grows, moving the nerve to the inferior part of the goiter (Figure 2). Pulling the thyroid mass out of the mediastinum at the beginning of the operation is likely to overstretch the RLN imbedded in the inferior part of the goiter. In retrosternal goiters, extensive traction from the mediastinum should be avoided until the goiter is mobilized (52,53). All structures adherent to the inferior part of the goiter should be mapped using IONM. In retrosternal redo surgery, an inferior approach to the RLN seems optimal (44). Once identified, the RLN should be followed throughout its course and freed from the scar tissue under IONM guidance, with postoperative confirmation of RLN functionality (25).
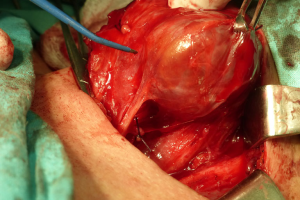
The RLN embedded in a scar in reoperation
Scarring from the first intervention is the main obstacle during reoperations (16-19,33,34) (Figure 3). As we have pointed out, it makes it difficult to identify and to dissect the RLN, and the operating field is much more prone for bleeding than in first intervention. Visual identification of the RLN may be difficult or even impossible by inexperienced personnel. Extensive scarring is usually seen shortly after primary thyroid operations, in particular when completing a total thyroidectomy in cases of thyroid cancer. Even when the scarring from the first operation is so massive that it is not possible to isolate the nerve, using IONM to map the outline of the nerve before any cutting can help the surgical team remove most of the remnant tissue around the nerve while minimizing damage to the nerve itself (16-19,33,34).
The extent of surgery in thyroid reoperations with IONM
Total thyroidectomy is recommended by many guidelines in both benign and malignant disorders (20,53,54). In recent years, we see fewer partial resections in cases of benign goiter, so the number of recurrent goiters is lower. The completeness of a total thyroidectomy needs to be ensured, not only because it can improve oncological outcomes in cancer patients, but also because each subsequent operation is associated with a higher incidence of nerve injury (14). After the first partial thyroid operation, the remnant tissue is usually close to Berry’s ligament or Zuckerkandl’s tubercle, where the RLN is at high risk of injury (40,50,53). The RLN is most commonly injured in the last 2 cm before entering the larynx (53,55). Neuromonitoring helps the surgeon achieve optimal exposure of the RLN in this region, making it possible to perform complete and secure resection in this difficult region (19).
In two groups of patients who underwent thyroid reoperations with or without IONM, the number of total thyroidectomies was higher in monitored operations (38% vs. 19.5% in procedures without IONM) (16). Similarly, in a study about thyroid reoperations by Barczytal thyroidectomies was higher in monitored operations (38% vs. 19.5% in procedures without IONM) (16). Similarly, in a study about thyroid reoperations by Barczyński et al., total thyroidectomies were performed significantly more often with IONM: 58.2% as opposed to 25.1% with only visual RLN identification (17).
Surgical strategy with IONM
Neuromonitoring should be carried out in accordance with the recommendations of the International Neural Monitoring Study Group (25,26).
The use of intermittent IONM allows the functionality of the RLN to be tested during surgery (25,44,47). Anatomical intactness of the nerve does not mean that the function of the nerve is preserved. In case of a decrease in amplitude by more than 30–50% from the baseline, surgeons can stop the procedure, reduce the traction and continue dissection after spontaneous EMG signal recovery.
Electric vagal nerve testing at the end of the procedure provides information about nerve functionality, which can influence surgical strategy as the operation proceeds. In case of a loss of signal on the dominant side of the neck operated on first, a staged thyroidectomy should be considered in order to prevent bilateral nerve injuries. Staged thyroidectomies are gaining acceptance among surgeons, particularly in redo surgery (56,57).
The prevalence of RLN injury in reoperations with IONM
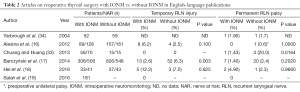
Despite lacking hard evidence that IONM can diminish prevalence of permanent vocal fold’s palsy as many as 95.7% of the respondents of the most recent international survey on the identification and neural monitoring of the EBSLN during thyroidectomy expressed their confidence in IONM and listed reoperative thyroid cases as the top indication far ahead all other clinical situations for utilization of this technique during thyroid surgery (58). There are only a few publications concerning the prevalence of RLN injury in reoperations with IONM, or comparing reoperations performed with or without IONM (16-19,33,34). Moreover, the power of the data are not sufficient to validate the differences, because of the small number of patients under consideration. Another problem is the lack of homogeneity among patients undergoing thyroid reoperations. There are different indications for reoperations; the extent of surgery ranges from lobectomy to the completion of a total resection; and the experience of the surgeons performing the operations are different as well. The prevalence of RLN injury during reoperations as reported in the available literature is shown in Table 2.
Full table
In a major retrospective cohort study from 2014, Barczyński et al. compared reoperations with and without IONM, and found that intraoperative neuromonitoring can reduce the prevalence of recurrent laryngeal nerve injury in thyroid reoperations (P=0.001). The study included 854 patients (1,326 RLNs at risk), and transient and permanent RLN injuries were found respectively in 13 (2.6%) and 7 (1.4%) nerves with IONM, as opposed to 52 (6.3%) and 20 (2.4%) nerves without IONM (BAR). IONM significantly decreased the incidence of transient RLN paresis compared with visualization alone (P=0.003); the prevalence of permanent RLN injury was also lower with IONM, but the difference was not statistically significant (P=0.202) (17). In 7.5% of the patients who underwent reoperations, staged thyroidectomy was performed due to intraoperative loss of signal. No bilateral RLN palsy was observed in the group with IONM. In one patient bilateral palsy was observed in the group that underwent reoperation with only RLN visualization, but unilateral palsy was observed after the first operation. It is worth emphasizing that in reoperations both the negative predictive value (NPV) and positive predictive value (PPV) of IONM were high: 99.6% and 78.3% respectively (17).
Chuang and Huang recommended the routine use of IONM in thyroid and parathyroid reoperations because the rate of RLN injury with IONM was 1.43%, as opposed to 20% without monitoring (P=0.0164) (33).
The results reported by Barczyński et al. and by Chuang and Huang differ from findings published earlier. In 2004, Yarbrough et al. considered 52 cervical reexploration procedures, and showed that intraoperative monitoring of the RLN in reoperative neck surgery can be performed safely, but did not decrease RLN complications. In that study, there was a similar rate of complications in both groups: 1.9% with IONM vs. 1.7% without IONM. Yarbrough et al. concluded that thyroid reexplorations could be performed safely, but experience and routine nerve exposure remain crucial to the minimization of RLN complications (34). Similarly, Alesina et al. did not observe significant differences in the prevalence of RLN injuries between 91 operations with IONM and 159 procedures with direct nerve visualization (16). The overall incidence of postoperative transient nerve palsy was 4.1%, and it was not significantly different between the groups (P=0.1). However, this study was influenced by selection bias, as the decision to use IONM was dependent on the availability of the equipment and the individual surgeon’s preferences considering the planned extent of the surgical procedure. In the neuromonitoring group, there were twice as many total thyroid operations, which is an additional risk factor for complications (16). Moreover, the studies by Yarbrough et al. and Alesina et al. were both based on relatively small groups of patients, and statistical validation of small differences in the prevalence of RLN injury require more RLNs at risk.
The next study we found regarding the use of intermittent IONM in thyroid reoperations was from 2015 (18). It was a small randomized single-surgeon study involving 70 patients; 33 of them (41 RLNs at risk) underwent reoperations with IONM, and 37 (43 RLNs at risk) were enrolled in the control group, who were operated on without IONM. In the IONM group the incidence of temporary RLN paralysis was 12.2%, and the rate of permanent RLN paralysis was 4.9%, compared with 7.0% and 2.3% in the control group (P=0.658, P=0.966, respectively) (18). The limitations of this study were the small sample and the fact that it was a single-surgeon study.
In 2015, the Laryngoscope published an interesting review article by Salari et al. about the use of IONM in recurrent thyroid cancer. The study involved 181 patients undergoing reoperation for local recurrent thyroid cancer, all performed by the same surgeon and all using IONM. Fourteen percent of the patients presented with permanent vocal cord paresis (VCP) after the first operation. Interestingly, none developed temporary or permanent VCP after the thyroid reoperation. Salari et al. pointed out that techniques such as IONM allow the identification of the vagus nerve at the initial phase of surgery, and the identification of the RLN inferiorly in the tracheoesophageal groove can help reduce the morbidity of central compartment dissections. The study also demonstrated that neuromonitored reoperative thyroid surgery in a large cohort of papillary thyroid cancer patients is effective, with a robust response in thyroglobulin (in this study the sTG level was undetectable in 58% of the PTC patients postoperatively), while at the same time it is safe, with a low rate of complications, even among the 14% of the patients who had unilateral palsy before surgery (19).
Conclusions
Extensive knowledge of RLN anatomy, routine visual RLN identification, experience and training are standard requirements for RLN management during thyroidectomy (42,44,46,47). But in reoperative thyroid surgery, even experienced surgeons can have difficulty in identifying the nerve and dissecting it from scar tissue (11,12,14,16-19,33). Whether intermittent IONM can provide additional benefits in thyroid reoperations is still controversial among surgeons (16,17). If we asses only the prevalence of RLN injury in reoperations with or without IONM, the results differ from study to study. On the other hand, in each of the publications mentioned in this review we found that IONM provides more information about RLN anatomy and its branches, and helped predict postoperative RLN function. In all the studies, the value of IONM in dissecting the RLN from scar tissue or mapping a distorted nerve is emphasized.
One problem in evaluating redo surgery with IONM is the fact that most of the articles have some limitations: small groups of patients, differences in the amount of experience the surgeons have, different operating techniques; and we found only one randomized study. In the future, multicenter prospective studies with large sample sizes are needed to further assess the role of intermittent IONM in thyroid reoperations. Despite lacking hard evidence that IONM can diminish prevalence of permanent vocal fold’s palsy as many as 95.7% of the respondents of the most recent international survey on the identification and neural monitoring of the EBSLN during thyroidectomy expressed their confidence in IONM and listed reoperative thyroid cases as the top indication far ahead all other clinical situations for utilization of this technique during thyroid surgery (58).
The introduction of continuous neuromonitoring could also represent a promising innovation in redo surgery.
In the available literature we did not find any analysis of the cost-effectiveness of routine use of IONM in thyroid reoperations, and this topic should be clarified as well.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reeve TS, Delbridge L, Brady P, et al. Secondary thyroidectomy: a twenty-year experience. World J Surg 1988;12:449-53. [Crossref] [PubMed]
- Levin KE, Clark AH, Duh QY, et al. Reoperative thyroid surgery. Surgery 1992;111:604-9. [PubMed]
- Seiler CA, Glaser C, Wagner HE. Thyroid gland surgery in an endemic region. World J Surg 1996;20:593-6; discussion 596-7. [Crossref] [PubMed]
- Chao TC, Jeng LB, Lin JD, et al. Reoperative thyroid surgery. World J Surg 1997;21:644-7. [Crossref] [PubMed]
- Peix JL, Van Box Som P, Olagne E, et al. Results of reoperations for goiter. Ann Chir 1997;51:217-21. [PubMed]
- Makeieff M, Rubinstein P, Youssef B, et al. Repeat surgery for thyroid nodules (excluding cancer and hyperthyroidism). Ann Chir 1998;52:970-7. [PubMed]
- Wilson DB, Staren ED, Prinz RA. Thyroid reoperations: indications and risks. Am Surg 1998;64:674-8; discussion 678-9. [PubMed]
- Menegaux F, Turpin G, Dahman M, et al. Secondary thyroidectomy in patients with prior thyroid surgery for benign disease: a study of 203 cases. Surgery 1999;126:479-83. [Crossref] [PubMed]
- Müller PE, Jakoby R, Heinert G, et al. Surgery for recurrent goitre: its complications and their risk factors. Eur J Surg 2001;167:816-21. [Crossref] [PubMed]
- Gibelin H, Sierra M, Mothes D, et al. Risk factors for recurrent nodular goiter after thyroidectomy for benign disease: case-control study of 244 patients. World J Surg 2004;28:1079-82. [Crossref] [PubMed]
- Lefevre JH, Tresallet C, Leenhardt L, et al. Reoperative surgery for thyroid disease. Langenbecks Arch Surg 2007;392:685-91. [Crossref] [PubMed]
- Calò PG, Pisano G, Medas F, et al. Risk factors in reoperative thyroid surgery for recurrent goitre: our experience. G Chir 2012;33:335-8. [PubMed]
- Kurmann A, Herden U, Schmid SW, et al. Morbidity rate of reoperation in thyroid surgery: a different point of view. Swiss Med Wkly 2012;142:w13643. [PubMed]
- Hardman JC, Smith JA, Nankivell P, et al. Re-operative thyroid surgery: a 20-year prospective cohort study at a tertiary referral centre. Eur Arch Otorhinolaryngol 2015;272:1503-8. [Crossref] [PubMed]
- Miccoli P, Frustaci G, Fosso A, et al. Surgery for recurrent goiter: complication rate and role of the thyroid-stimulating hormone-suppressive therapy after the first operation. Langenbecks Arch Surg 2015;400:253-8. [Crossref] [PubMed]
- Alesina PF, Rolfs T, Hommeltenberg S, et al. Intraoperative neuromonitoring does not reduce the incidence of recurrent laryngeal nerve palsy in thyroid reoperations: results of a retrospective comparative analysis. World J Surg 2012;36:1348-53. [Crossref] [PubMed]
- Barczyński M, Konturek A, Pragacz K, et al. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg 2014;38:599-606. [Crossref] [PubMed]
- Hei H, Zhou B, Qin J, et al. Intermittent intraoperative nerve monitoring in thyroid reoperations: Preliminary results of a randomized, single-surgeon study. Head Neck 2016;38 Suppl 1:E1993-7. [Crossref] [PubMed]
- Salari B, Ren Y, Kamani D, et al. Revision neural monitored surgery for recurrent thyroid cancer: Safety and thyroglobulin response. Laryngoscope 2016;126:1020-5. [Crossref] [PubMed]
- Dralle H, Stang A, Sekulla C, et al. Surgery for benign goiter in Germany: fewer operations, changed resectional strategy, fewer complications. Chirurg 2014;85:236-45. [Crossref] [PubMed]
- Barczyński M, Konturek A, Stopa M, et al. Total thyroidectomy for benign thyroid disease: is it really worthwhile? Ann Surg 2011;254:724-29; discussion 729-30. [Crossref] [PubMed]
- Barczyński M, Konturek A, Hubalewska-Dydejczyk A, et al. Five-year follow-up of a randomized clinical trial of total thyroidectomy versus Dunhill operation versus bilateral subtotal thyroidectomy for multinodular nontoxic goiter. World J Surg 2010;34:1203-13. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Jatzko GR, Lisborg PH, Müller MG, et al. Recurrent nerve palsy after thyroid operations--principal nerve identification and a literature review. Surgery 1994;115:139-44. [PubMed]
- Randolph GW, Dralle H, International Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121 Suppl 1:S1-16. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123 Suppl 4:S1-14. [Crossref] [PubMed]
- Dionigi G, Bacuzzi A, Boni L, et al. What is the learning curve for intraoperative neuromonitoring in thyroid surgery? Int J Surg 2008;6 Suppl 1:S7-12. [Crossref] [PubMed]
- Dralle H. Surgical assessment of complications after thyroid gland operations. Chirurg 2015;86:70-7. [Crossref] [PubMed]
- Snyder SK, Hendricks JC. Intraoperative neurophysiology testing of the recurrent laryngeal nerve: plaudits and pitfalls. Surgery 2005;138:1183-91; discussion 1191-2. [Crossref] [PubMed]
- Barczynski M, Konturek A, Cichon S. Value of the intraoperative neuromonitoring in surgery for thyroid cancer in identification and prognosis of function of the recurrent laryngeal nerves. Endokrynol Pol 2006;57:343-6. [PubMed]
- Wojtczak B, Sutkowski K, Kaliszewski K, et al. Experience with intraoperative neuromonitoring of the recurrent laryngeal nerve improves surgical skills and outcomes of non-monitored thyroidectomy. Langenbecks Arch Surg 2016. [Epub ahead of print]. [PubMed]
- Wojtczak B, Kaliszewski K, Sutkowski K, et al. The learning curve for intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Langenbecks Arch Surg 2016. [Epub ahead of print]. [PubMed]
- Chuang YC, Huang SM. Protective effect of intraoperative nerve monitoring against recurrent laryngeal nerve injury during re-exploration of the thyroid. World J Surg Oncol 2013;11:94. [Crossref] [PubMed]
- Yarbrough DE, Thompson GB, Kasperbauer JL, et al. Intraoperative electromyographic monitoring of the recurrent laryngeal nerve in reoperative thyroid and parathyroid surgery. Surgery 2004;136:1107-15. [Crossref] [PubMed]
- Lahey FH. Routine dissection and demonstration recurrent laryngeal nerves in subtotal thyroidectomy. Surg Gynecol Obstet 1938;66:775-7.
- Hermann M, Alk G, Roka R, et al. Laryngeal recurrent nerve injury in surgery for benign thyroid diseases: effect of nerve dissection and impact of individual surgeon in more than 27,000 nerves at risk. Ann Surg 2002;235:261-8. [Crossref] [PubMed]
- Serpell JW, Lee JC, Yeung MJ, et al. Differential recurrent laryngeal nerve palsy rates after thyroidectomy. Surgery 2014;156:1157-66. [Crossref] [PubMed]
- Shedd DP, Burget GC. Identification of the recurrent laryngeal nerve. Arch Surg 1966;92:861-4. [Crossref] [PubMed]
- Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 2009;96:240-6. [Crossref] [PubMed]
- Hamelmann WH, Meyer T, Timm S, et al. A Critical Estimation of Intraoperative Neuromonitoring (IONM) in Thyroid Surgery. Zentralbl Chir 2002;127:409-13. [Crossref] [PubMed]
- Jonas J. Reliabilty of intraoperative recurrent laryngeal nerve monitoring in thyroid surgery. Zentralbl Chir 2002;127:404-8. [Crossref] [PubMed]
- Chiang FY, Lu IC, Tsai CJ, et al. Does extensive dissection of recurrent laryngeal nerve during thyroid operation increase the risk of nerve injury? Evidence from the application of intraoperative neuromonitoring. Am J Otolaryngol 2011;32:499-503. [Crossref] [PubMed]
- Serpell JW, Yeung MJ, Grodski S. The motor fibers of the recurrent laryngeal nerve are located in the anterior extralaryngeal branch. Ann Surg 2009;249:648-52. [Crossref] [PubMed]
- Randolph G. Surgical anatomy of recurrent laryngeal nerve. In: Randolph GW. editor. Surgery of the thyroid and parathyroid glands. Philadelphia: Saunders, 2013.
- Chiang FY, Lu IC, Chen HC, et al. Intraoperative neuromonitoring for early localization and identification of recurrent laryngeal nerve during thyroid surgery. Kaohsiung J Med Sci 2010;26:633-9. [Crossref] [PubMed]
- Chiang FY, Lu IC, Chen HC, et al. Anatomical variations of recurrent laryngeal nerve during thyroid surgery: how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci 2010;26:575-83. [Crossref] [PubMed]
- Dionigi G, Barczynski M, Chiang FY, et al. Why monitor the recurrent laryngeal nerve in thyroid surgery? J Endocrinol Invest 2010;33:819-22. [Crossref] [PubMed]
- Wu CW, Lu IC, Randolph GW, et al. Investigation of optimal intensity and safety of electrical nerve stimulation during intraoperative neuromonitoring of the recurrent laryngeal nerve: a prospective porcine model. Head Neck 2010;32:1295-301. [Crossref] [PubMed]
- Dolezel R, Jarosek J, Hana L, et al. Clinical relevance and surgical anatomy of non-recurrent laryngeal nerve: 7 year experience. Surg Radiol Anat 2015;37:321-5. [Crossref] [PubMed]
- Mehanna R, Murphy MS, Sheahan P. Thyroid tubercle of zuckerkandl is more consistently present and larger on the right: a prospective series. Eur Thyroid J 2014;3:38-42. [PubMed]
- Rui Sheng Y, Chong Xi R. Surgical approach and technique in retrosternal goiter: Case report and review of the literature. Ann Med Surg (Lond) 2015;5:90-2. [Crossref] [PubMed]
- Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery 2014;155:329-39. [Crossref] [PubMed]
- Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. [Crossref] [PubMed]
- Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg 2008;32:1301-12. [Crossref] [PubMed]
- Snyder SK, Lairmore TC, Hendricks JC, et al. Elucidating mechanisms of recurrent laryngeal nerve injury during thyroidectomy and parathyroidectomy. J Am Coll Surg 2008;206:123-30. [Crossref] [PubMed]
- Dionigi G, Frattini F. Staged thyroidectomy: time to consider intraoperative neuromonitoring as standard of care. Thyroid 2013;23:906-8. [Crossref] [PubMed]
- Fontenot TE, Randolph GW, Setton TE, et al. Does intraoperative nerve monitoring reliably aid in staging of total thyroidectomies? Laryngoscope 2015;125:2232-5. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea C, et al. International survey on the identification and neural monitoring of the EBSLN during thyroidectomy. Laryngoscope 2016;126:285-91. [Crossref] [PubMed]