
Lenvatinib and selpercatinib successfully treated RET fusion gene-positive papillary thyroid carcinoma cardiac metastases: a case report
Highlight box
Key findings
• This is the first case of cardiac metastasis of papillary thyroid carcinoma treated successfully with systemic therapy.
What is known and what is new?
• Cardiac metastasis of thyroid cancer has an extremely poor prognosis, and only patients who undergo cardiac surgery can survive for a long time.
• Papillary thyroid carcinoma with cardiac metastasis can be shrunk and controlled by systemic therapy such as lenvatinib or selpercatinib.
What is the implication, and what should change now?
• Systemic therapy has emerged as a significant alternative in patients with difficult to resect cardiac metastases.
IntroductionOther Section
The most common type of thyroid cancer, papillary thyroid carcinoma (PTC), has a good prognosis. Patients under the age of 55 years have a <5% risk of thyroid cancer-related death at 10 years after diagnosis even if they have distant metastases (1). Surgery and radioactive iodine (RAI) therapy have been the mainstays of treatment for many years, and they can manage many cases of PTC. However, in recent years, molecular-targeted drugs have been developed, and treatment strategies are changing. Multiple kinase inhibitors such as sorafenib and lenvatinib have demonstrated prolongation of progression-free survival in RAI-refractory progressive differentiated thyroid carcinoma, thereby becoming the first-line therapy (2,3). Furthermore, comprehensive cancer genomic profiling tests have confirmed that many actionable genetic alterations, such as BRAF, RET, and NTRK, also occur in advanced thyroid cancer (4). Certain RET inhibitors, including selpercatinib and pralsetinib, have high efficacy, especially for thyroid tumors with RET alterations (5,6). Advanced thyroid tumors are increasingly being treated with these drug therapies based on the driver genes. However, cardiac metastasis from thyroid cancer is rare and has an extremely poor prognosis. A systematic review found that median survival from detection of cardiac metastases was 12.5 weeks, and all patients with at least short-term survival underwent cardiac surgery (7,8). Heart surgery was once the sole option for treating thyroid cancer cardiac metastasis; however, this option is not always available. To the best of our knowledge, there are no reports of PTC-related systemic therapy for cardiac metastasis. Therefore, we describe a case of effective systemic therapy for multiple cardiac metastases. We present this case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-252/rc).
Case presentationOther Section
A 53-year-old woman presented with cough, right chest pain, and difficulty lifting her right shoulder. She had a history of PTC with pulmonary metastasis when she was 15 years old. She underwent total thyroidectomy followed by three rounds of RAI therapy. Thereafter, a family doctor had prescribed her levothyroxine for >20 years without imaging tests. She did not have a family history of cancer and her only medical history was thyroid cancer and uterine fibroids. She was taking 125 µg/day of levothyroxine and 1 µg/day of alfacalcidol.
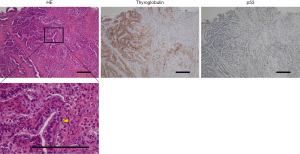
Metastases to the lung, chest wall, liver, heart, and lymph nodes in the neck, mediastinum, and right axilla were discovered on computed tomography (CT) (Figure 1, Table 1). PTC recurrence was discovered during core needle biopsy of the tumor in the right chest wall, wherein cells with intranuclear inclusions grew in a papillary manner. Necrotic foci were found in some regions. Thyroid transcription factor-1, paired box 8, and thyroglobulin were positive and p53 was partially positive on immunohistochemical staining (Figure 2). Electrocardiogram indicated total right bundle branch block; the left ventricular septum and right ventricular apex had mass lesions according to echocardiography (Figure 3A,3B). The use of cardiac magnetic resonance imaging (CMR) revealed masses at the ventricular septum and the apex (Figure 3C,3D). Thyroid stimulating hormone (TSH) and thyroglobulin levels in blood were 0.053 µIU/mL (normal range, 0.61–4.23 µIU/mL) and 851 ng/mL (normal range, 0–35.1 ng/mL) respectively, whereas antithyroglobulin antibody levels were below the detection threshold. Derived from the presence of multiple masses and hyperthyroglobulinemia, we diagnosed any mass lesions are thyroid carcinoma metastases.

Table 1
| Item | Timing of examination (after the initiation of treatment) | ||||
|---|---|---|---|---|---|
| First visit | 3 weeks | 8 weeks | 13 weeks | 20 weeks | |
| Longest diameter of metastatic site (mm) | |||||
| Right axilla lymph node | 39.3 | 29.3 | 28.9 | 26.6 | 25.1 |
| Mediastinum lymph node | 20.8 | 17.3 | 14.2 | 14.3 | 12.4 |
| Lung | 50.2 | 40.9 | 39.2 | 36.6 | 35.5 |
| Chest wall | 86.3 | 70.7 | 64.4 | 58.3 | 53.0 |
| Liver | 52.8 | 46.1 | 45.3 | 40.9 | 34.6 |
| Heart | 48.5 | 39.3 | 35.7 | 35.1 | 35.1 |
| Blood test | |||||
| TSH (μIU/mL) | 0.053 | ND | 3.959 | 2.322 | 0.104 |
| Tg (ng/mL) | 851 | ND | 194 | 68.1 | 63.9 |
Lymph nodes are measured in short axis. TSH, thyroid stimulating hormone; ND, no data.

Based on the presence of cardiac metastasis and strong clinical symptoms, the patient’s condition was assumed to be fatal, and lenvatinib (24 mg per day) was started immediately. Three weeks after starting lenvatinib, CT scan showed that most of the metastatic lesions were decreasing in size. Moreover, the Oncomine Dx Target Test system, a gene panel test using a next-generation sequencer, detected the ERC1-RET fusion gene from the biopsy specimen. Fatal adverse-events due to major hemorrhage have been reported in lenvatinib. Therefore, we changed our treatment from lenvatinib to selpercatinib. Initially, selpercatinib (160 mg) was administered twice daily. Seven days after the initiation of selpercatinib, the electrocardiogram QT interval corrected with the Bazett formula was prolonged to 502 msec. Subsequently, selpercatinib was reduced to 120 mg twice daily after one week of withdrawal. Ten weeks after the selpercatinib treatment began, CT scan showed that the tumors had shrunk further (Figure 1), and blood thyroglobulin level decreased to 68.1 ng/mL. Initial symptoms, such as cough and right chest pain, improved. Hypertension (grade 3, Common Terminology Criteria for Adverse Events version 5.0), proteinuria (grade 3), platelet count decreased (grade 2), and cheilitis (grade 1) were observed after initiation of lenvatinib. Adverse events other than hypertension were resolved with the discontinuation of lenvatinib. Although dry mouth (grade 1) and dysgeusia (grade 2) occurred after starting selpercatinib, these adverse events were manageable with supportive therapy. 30 weeks after the initiation of systemic therapy, she is alive and without major obstacles to daily life.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
DiscussionOther Section
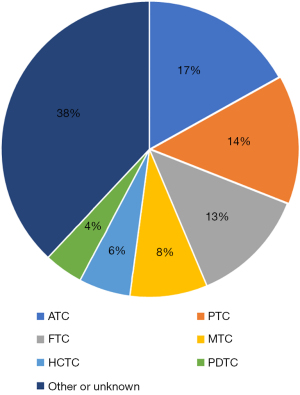
PTC often results in distant metastases in the lungs and bones. It may sometimes lead to metastases in the brain or skin; however, cardiac metastases are extremely rare (1,7). In a literature review of cardiac metastasis published in 2011, 55 cases over a 130-year period were found. The most common histologic type of thyroid carcinoma with cardiac metastasis was anaplastic carcinoma, but it was also found in papillary, follicular, poorly differentiated, and medullary carcinomas. Average survival from the detection of cardiac metastases was 12.5 weeks. All patients with at least short-term survival had undergone cardiac surgery (8). Therefore, surgery is recommended in operable cases. Since 2011, 16 cases of cardiac metastasis from thyroid cancer have been reported, and many of the long-term survivors also underwent cardiac surgery (9-22) (Table 2). Combined with the literature review in 2011, the most common histologic types were ATC in 12 cases, followed by PTC in 10 and follicular thyroid carcinoma (FTC) in 9 cases (Figure 4). In contrast, systemic therapy for thyroid cancer has become well-established in recent years, and its use in two instances of cardiac metastasis from medullary thyroid carcinoma has been reported. Vandetanib therapy was successful in patients for 4 and >11 years (21). To our knowledge, however, only vandetanib therapy for medullary carcinoma has been shown to be effective in treating thyroid cancer with cardiac metastasis.
Table 2
| Case | Age (years) | Sex | Histology | Location of cardiac metastasis | Treatment for cardiac metastasis | Outcome | Year | Citation |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | PDTC with clear cells features | Right ventricle | Heart surgery | Death; 3 weeks | 2011 | (9) |
| 2 | 54 | F | PTC, tall cell variant | Right atrium | Heart surgery | Alive; 2 years | 2012 | (10) |
| 3 | 76 | M | PTC | Apical septum | UN | UN | 2013 | (11) |
| 4 | 52 | M | HCTC | Left atrium | UN | UN | 2014 | (12) |
| 5 | 57 | F | Squamous cell carcinoma | Ventricular septum | Paclitaxel and concurrent irradiation, lenvatinib | Death; 2 months | 2017 | (13) |
| 6 | 58 | UN | Clear cell thyroid cancer | Right atrium | Heart surgery | Alive | 2017 | (14) |
| 7 | 52 | F | FTC, oncocytic variant | Apical septum | Heart surgery | Alive | 2018 | (15) |
| 8 | 49 | F | MTC | Atrial septum | UN | UN | 2020 | (16) |
| 9 | 70 | F | FTC | Left atrium | Heart surgery | Alive | 2020 | (17) |
| 10 | 69 | F | ATC | Right ventricle | UN | UN | 2021 | (18) |
| 11 | 61 | M | ATC | Right atrium | UN | UN | 2021 | (18) |
| 12 | 57 | F | FTC | Left ventricle | Heart surgery | Alive | 2021 | (19) |
| 13 | 63 | F | HCTC | Right atrial septum | Heart surgery | Alive; 8 years | 2021 | (20) |
| 14 | 45 | F | MTC | Right ventricular apex | Vandetanib | Alive; 13 years | 2021 | (21) |
| 15 | 63 | F | MTC | Right ventricle and atrium | Vandetanib, heart surgery | Death; 6 years | 2021 | (21) |
| 16 | 76 | F | MTC | Right ventricle | Heart surgery | Death; 40 months | 2022 | (22) |
M, male; PDTC, poorly differentiated thyroid carcinoma; F, female; PTC, papillary thyroid carcinoma; UN, unknown; HCTC, Hurthle cell thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; ATC, anaplastic thyroid carcinoma.
Although cardiac metastases are often not recognized or checked for, they are likely to be found incidentally on CT scans. Echocardiography and CMR are useful methods for scrutinizing cardiac metastasis (8). CMR provides high-resolution assessment of anatomical sites and cardiac function. In a report examining CMR in various carcinomas, excluding thyroid cancer, 22% of cardiac metastatic cases involved multiple cardiac chambers. Additionally, right-sided chamber involvement was prevalent in hematogenous and lymphatic metastases, whereas the placement of the pericardium was common in direct invasion (23). Further, medullary thyroid cancer diagnosis using immuno-positron emission tomography scan with anti-carcinoembryonic antigen bispecific antibody and gallium-68-labeled peptide has been reported to be successful (21).
In this case, it was necessary to start medical treatment as soon as possible because the condition was fatal. As lenvatinib is the first-line drug for papillary thyroid cancer with an unknown driver gene, it was started immediately. We switched to selpercatinib as the RET fusion gene was found to be the driving gene. We considered that the risk of fatal major bleeding events was lower with selpercatinib than with lenvatinib because there were no adverse events due to vascular rupture in the clinical trial of selpercatinib (5). Lenvatinib has been associated with fatal hemorrhagic episodes. In a single-center retrospective study, cutaneous fistulas formed in 34.4% of patients with ATC and 8.9% of those with DTC after lenvatinib therapy, of which 16.6% experienced major bleeding (24). However, no adverse events related to cardiac metastasis have been reported with lenvatinib. The response rate for thyroid cancer in clinical trials is 64.7% for lenvatinib and 79% for selpercatinib, both of which are very high (3,5). Selpercatinib has a known resistance mechanism (25), but the resistance mechanism for lenvatinib is unknown. Lenvatinib is a multikinase inhibitor (26) and is unlikely to develop resistance. In this case, after starting treatment with lenvatinib and selpercatinib, the tumor shrank and the symptoms improved. Therefore, it is considered that the treatments were effective, at least in the short term. However, due to the short follow-up period, it is not known whether the systemic therapy improves long-term prognosis.
ConclusionsOther Section
This is the first case of cardiac metastasis of PTC treated successfully with systemic therapy. Conventionally, cardiac surgery was the primary treatment for cardiac metastasis; now, systemic therapy has emerged as a significant alternative. It is important to choose therapeutic drugs based on gene and adverse-event profiling.
AcknowledgmentsOther Section
The authors would like to thank Enago (https://www.enago.jp/) for the English language review.
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-252/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-252/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-252/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Schlumberger M, Leboulleux S. Current practice in patients with differentiated thyroid cancer. Nat Rev Endocrinol 2021;17:176-88. [Crossref] [PubMed]
- Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384:319-28. [Crossref] [PubMed]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. [Crossref] [PubMed]
- Pozdeyev N, Gay LM, Sokol ES, et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin Cancer Res 2018;24:3059-68. [Crossref] [PubMed]
- Wirth LJ, Sherman E, Robinson B, et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med 2020;383:825-35. [Crossref] [PubMed]
- Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 2021;9:491-501. [Crossref] [PubMed]
- Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892-9. [Crossref] [PubMed]
- Catford SR, Lee KT, Pace MD, et al. Cardiac metastasis from thyroid carcinoma. Thyroid 2011;21:855-66. [Crossref] [PubMed]
- Pazzano V, Narducci ML, Santangeli P, et al. Poorly differentiated thyroid carcinoma with cardiac metastasis and pulmonary embolism as first clinical presentation: case report and review of the literature. J Endocrinol Invest 2011;34:164-5. [Crossref] [PubMed]
- Kaul S, Tulchinsky M, Campbell DB, et al. Isolated cardiac metastasis from papillary thyroid cancer: prolonged survival with late diagnosis related to inadequate positron emission tomography preparation. Thyroid 2012;22:443-4. [Crossref] [PubMed]
- Yarmohammadi H, Tacher V, Faulhaber PF, et al. Imaging of dedifferentiated papillary thyroid carcinoma with left ventricular metastasis: A rare presentation of papillary thyroid metastatic disease. J Cancer Res Ther 2013;9:490-2. [Crossref] [PubMed]
- Giovanella L, Treglia G, Ceriani L, et al. Left atrial metastasis of Hürthle-cell thyroid carcinoma mimicking myxoma. J Nucl Cardiol 2014;21:406-7. [Crossref] [PubMed]
- Yoshihiro T, Tsuchihashi K, Kusaba H, et al. Cardiac metastasis of squamous cell carcinoma of the thyroid gland with severe disseminated intravascular coagulation: A case report. Mol Clin Oncol 2017;6:91-5. [Crossref] [PubMed]
- Martínez-Milla J, Cortés M, Lara JI, et al. Cardiac metastasis of clear cell thyroid cancer. J Nucl Cardiol 2017;24:2037-9. [Crossref] [PubMed]
- Stegger L, Rahbar K, Schülke C, et al. A Cardiac Metastasis of Follicular Thyroid Carcinoma With Partly Squamous Cell Differentiation: Detection in FDG-PET Preceded Visibility on Echocardiography and MRI by More Than a Year. Clin Nucl Med 2018;43:e473-4. [Crossref] [PubMed]
- El Ghannudi S, Germain P, Schneegans O, et al. Cardiac metastasis from medullary thyroid carcinoma: insights from multimodal molecular imaging and magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2020;21:231-2. [PubMed]
- Peng CH, Tai HC, Chung JY, et al. Thyroid Cancer With Cardiac Metastasis Presented With New Onset of Atrial Fibrillation. J Acute Med 2020;10:45-7. [PubMed]
- Cianciulli TF, Argento LV, Saccheri MC, et al. Cardiac metastasis from primary anaplastic thyroid carcinoma. Medicina (B Aires) 2021;81:637-40. [PubMed]
- Zieliński J, Kołsut P, Kuśmierczyk M, et al. Left ventricular wall invaded by thyroid cancer metastasis. Kardiol Pol 2021;79:89-90. [Crossref] [PubMed]
- Blossey RD, Kleine-Döpke D, Ringe KI, et al. Recurrent Hurthle cell thyroid carcinoma does not preclude long-term survival: a case report and review of the literature. J Med Case Rep 2021;15:399. [Crossref] [PubMed]
- Buffet C, Leboulleux S, Kraeber-Bodéré F, et al. Cardiac Metastasis from Medullary Thyroid Cancers with Long-Term Survival under Vandetanib. Eur Thyroid J 2021;10:517-22. [Crossref] [PubMed]
- Tricard J, Chermat A, Abdelkafi E, et al. Giant intracardiac medullary thyroid cancer metastasis. J Card Surg 2022;37:5455-6. [Crossref] [PubMed]
- Pun SC, Plodkowski A, Matasar MJ, et al. Pattern and Prognostic Implications of Cardiac Metastases Among Patients With Advanced Systemic Cancer Assessed With Cardiac Magnetic Resonance Imaging. J Am Heart Assoc 2016;5:e003368. [Crossref] [PubMed]
- Iwasaki H, Toda S, Murayama D, et al. Relationship between adverse events associated with lenvatinib treatment for thyroid cancer and patient prognosis. Mol Clin Oncol 2021;14:28. [Crossref] [PubMed]
- Rosen EY, Won HH, Zheng Y, et al. The evolution of RET inhibitor resistance in RET-driven lung and thyroid cancers. Nat Commun 2022;13:1450. [Crossref] [PubMed]
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 2008;122:664-71. [Crossref] [PubMed]


