
Right gasless transaxillary endoscopic total thyroidectomy (RGTETT) with video: our experience with the posterior approach: Lei’s seven-sinking method
Highlight box
Surgical highlights
• Our modified posterior approach uses a retractor to lift the muscle and thyroid, which can not only solve the problem of the visual blind area but also provide good tension and free-up hands for operation. The posterior approach can remove the central tissue with the gland, which avoids the incomplete cleaning caused by fat retraction after adenectomy. The posterior approach can better expose the recurrent laryngeal nerve and superior laryngeal nerve, and it is easier to address the root of the thyroid gland.
What is conventional and what is novel/modified?
• The conventional total thyroidectomy is open surgery.
• Our modified posterior approach uses a retractor to lift the muscle and thyroid.
What is the implication, and what should change now?
• This posterior approach could be a viable alternative to open surgery in well-selected patients for transaxillary endoscopic total thyroidectomy.
Introduction
The incidence of papillary thyroid cancer is increasing annually (1). Due to the majority of patients being female, there are also high requirements for surgical cosmetic effects. Various endoscopic thyroid surgeries are widely used in clinical practice to avoid neck incisions. Gasless transaxillary endoscopic thyroidectomy is developing very rapidly with satisfactory surgical outcomes and cosmetic appearance (2-5). However, it still faces the problem of space limitations and limited angles while dealing with the upper pole of the thyroid and the difficulty of exposing and accessing the contralateral lobe using conventional endoscopic instruments.
Our modified posterior approach uses a retractor to lift the muscle and thyroid together with the central lymph nodes, which can not only solve the problem of the visual blind area but also provide good tension and free-up hands for operation. The posterior approach can remove the central tissue with the gland, which avoids the incomplete cleaning caused by fat retraction after adenectomy. At the same time, the posterior approach can better expose the recurrent laryngeal nerve (RLN) and superior laryngeal nerve, and it is easier to address the root of the thyroid gland. Our previous research has confirmed that a novel anatomy-based five-settlement method for endoscopic thyroid lobectomy and ipsilateral central compartment neck dissection via a gasless unilateral axillary approach is safe and effective (6). We also found that for thyroid surgeons with prior experience, approximately 38 procedures could be considered to be the threshold for achieving proficiency in endoscopic thyroid lobectomy and ipsilateral central compartment neck dissection via gasless unilateral axillary with the posterior approach (7). More procedures are obviously needed for right gasless transaxillary endoscopic total thyroidectomy (RGTETT).
On the previous basis, we further explored the techniques and steps of using traditional endoscopic instruments to perform bilateral thyroid and central region surgeries through unilateral armpit. Total thyroidectomy and bilateral central lymph node dissection can be achieved relatively easily through the posterior approach and the operation steps of Lei’s seven-sinking method. Here, we summarize the surgical steps in detail through a case report video (see Video 1 for details) and schematic diagram of a modified gasless right transaxillary endoscopic thyroid surgery for bilateral low-risk thyroid cancer with the posterior approach. We present this article in accordance with the SUPER reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-204/rc).
Preoperative preparations and requirements
The patients underwent operation at Nanfang Hospital of Southern Medical University. We followed precise inclusion criteria: benign lesions such as thyroid nodules and adenomas, maximum diameter ≤6 cm; hyperthyroidism patients who needed surgery, and the swelling did not exceed the second degree; differentiated thyroid carcinoma with a maximum tumor diameter of less than 4 cm, no extraglandular invasion or only minor invasion of the anterior capsule, sternal thyroid muscle, cN0 or cN1a, and no mutual fused and fixed lymph nodes.
The exclusion criteria included body mass index (BMI) ≥24 kg/m2; cervicothoracic deformity and clavicle deformity; tumour that penetrated the posterior capsule or was located near the entrance of the RLN to the larynx; large and numerous metastatic lymph nodes with extracapsular invasion; and severe Hashimoto’s thyroiditis.
Step-by-step description
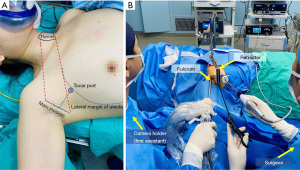
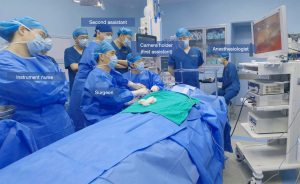
A 28-year-old female patient was referred for bilateral low-risk thyroid cancer with no underlying conditions, including diabetes, hypertension and coronary heart disease. The patient’s BMI was 17.9 kg/m2. All procedures performed in this study were in accordance with the ethical standards of the ethics committee of Nanfang Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The patient underwent general endotracheal anaesthesia. Prefashioned integrated paired left and right stainless-steel electrodes embedded within the endotracheal tube surface were used (NIM TriVantage® EMG Tube, Medtronic, Jacksonville, FL, USA). The electrodes were connected to the intraoperative neuromonitoring (IONM) station (NIM3.0, Medtronic). The patient was supine with a neck extension created by a pillow placed under the shoulders. The right arm was naturally extended 90°. After conventional disinfection and draping, the primary surgeon sat near the lower side of the right arm, and the assistant sat near the upper side of the right arm separately. A 5-cm main incision was made along the natural folds in the armpit (Figure 1A). Working space was created using electroscalpel by elevating a subcutaneous flap above the pectoralis major muscle. When separating the surface of the pectoralis major muscle, the second assistant was required to be on the opposite side of the surgeon, i.e., the patient’s left side, to lift the skin and subcutaneous tissue with a hook. When the electroscalpel could not continue separating the tissue, the second assistant installed the external retractor on the bedside opposite the primary surgeon and aligned it 1cm horizontally with the patient’s sternoclavicular joint. Then, the suspension hook was replaced, and a 5-mm trocar was placed 3–5 cm away from the incision and slightly below the anterior axillary line. A 30-degree rigid endoscope was inserted through the left end of the axillary incision, and then the division was performed under endoscopy (Figure 1B). Then, the first assistant and the surgeon could sit by the patient’s abducted arm. The first assistant sat above the arm, close to the patient’s head, and the surgeon sat below the arm, closer to the patient’s feet. The entire surgical process required the full cooperation of the instrument nurse and anesthesiologist (Figure 2). The anterior surface of the pectoralis major muscle was dissected using an ultrasonic scalpel under endoscopy until the sternocleidomastoid muscle (SCM) was exposed. First, the plier was placed in the corresponding position, and held with body, and then the retractor was adjusted. Under direct vision, the retractor was adjusted to the appropriate position following the plier and the plier was removed. After adjusting the retraction, the yellowish-white natural gap between the sternal heads of the SCM (SHSCM) and clavicular heads of the SCM (CHSCM) could be exposed naturally.
Step 1: sinking the CHSCM
After separating the clavicle, the SCM bundle was exposed, and the posterior edge of the SCM could be seen after a little separation. The yellow-white natural gap between the SHSCM and the CHSCM was exposed naturally or after being lifted by a retractor device. The fascial tissue was separated along the natural gap, and the SHSCM was lifted by moving the hook so as to achieve the first step of sinking the CHSCM (Figure 3).
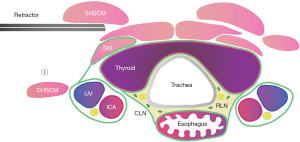
Step 2: sinking the cervical vascular sheath
The surface of the internal jugular vein is covered with multiple layers of deep cervical fascia, and the fascia of the neck muscles fuses in multiple layers, connecting the jugular sheath and the strap muscles (SMs). The jugular sheath could only be subsided by cutting those deep cervical fascia layer-by-layer. At the same time, on the inside of the common carotid artery, the deep cervical fascia that is continuous with the thyroid gland was detached, and it entered the prevertebral space vertically downwards. The prevertebral space was expanded and bare, and the inferior thyroid artery was detached. After the carotid sheath was submerged in the second step, the position of the retractor was adjusted and placed in the gap between the SMs. Through the force of the retractor, the thyroid gland was lifted up as a whole to create good tension. Because the thyroid gland was lifted, the tissues in the central area and the right esophagus were also pulled together (Figure 4). When separating the internal jugular vein, it is particularly important to pay attention to the use of an ultrasonic scalpel to avoid blindly damaging the internal jugular vein and causing massive bleeding. If there is significant bleeding from the internal jugular vein, immediate conversion to open surgery is needed.
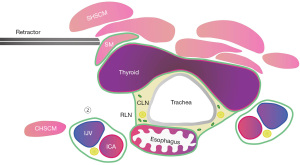
Step 3: sinking the right wall of the esophagus
Then, we sharply cut the posterior fascia of the esophagus, up to the outer side of the upper pole of the thyroid gland, down to the upper edge of the clavicle. During the separation process, the esophagus and thyroid should be fully identified to avoid accidentally injuring the esophagus. After sinking the right sidewall of the esophagus, the retractor was adjusted between the thyroid and the SMs, and the thyroid was further lifted to provide sufficient tension to better free the right RLN in the central tissue (Figure 5).

Step 4: sinking the right RLN
The lateral approach was used to provide the advantage of a better visual angle, the fascia was open, and the right RLN was located. In the process of freeing and sinking the RLN, the adipose lymph node tissue in the lower-VIb region was separated from the adipose lymph node tissue in the upper-VIa region. The adipose lymph node tissue in the VIa region was retained on the thyroid gland, while the adipose lymph node tissue in the VIb region was isolated (Figure 6). IONM was used during the operation to monitor the RLN in real time. A single-use, monopolar, 230 mm long stimulation probe (Medtronic) was used in this video for nerve stimulation. The probe was connected to the IONM station. The nerve monitoring standards developed by the International Neural Monitoring Study Group (INMSG) were applied (8). Following the four-step method, V1, R1, R2, and V2 were monitored and the signal values were recorded. The vagus nerve (VN) was stimulated (V1) prior to dissection of the thyroid, whereas the RLN was stimulated (R2) after initial identification (R1) and after completion of thyroidectomy. After thyroidectomy, the VN was stimulated again (V2). V1 and V2 were achieved by simply and gently applying the stimulating probe on the carotid sheath with a 3 mA stimulation intensity without carotid sheath dissection.

Step 5: sinking the trachea
Before starting to sink the trachea, the retractor was adjusted to fully lift the thyroid and VIa adipose lymph node tissue upwards, providing sufficient tension in the anterior tracheal space. Subsequently, an ultrasonic scalpel was used to fully free the surface of the trachea and operate closely against the trachea to avoid damaging the left RLN. During the process of sinking the trachea, the upper pole of the thyroid gland was handled. When disconnecting the upper pole of the thyroid gland and blood vessels, the ultrasonic scalpel was close to the upper pole of the thyroid gland to avoid damaging the superior laryngeal nerve. After fully sinking the trachea, the retractor was adjusted to the opposite gland to create a larger operating space and prepare for the next step of sinking the left RLN (Figure 7).
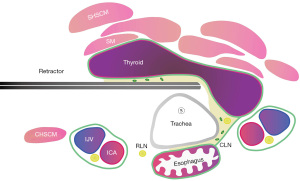
Step 6: sinking the left RLN
Below the left inferior pole of the thyroid gland, we separated and searched for the left RLN using separating forceps. After fully liberating the left RLN, the left RLN was sunk. At this point, the left adipose lymph node tissue remained on the left thyroid gland. During the process of sinking the left RLN, the upper pole of the left lobe of the thyroid gland was handled. During the process of sinking the left RLN, real-time neural monitoring is needed, and extra caution should be exercised when handling the entry point into the larynx (Figure 8). IONM can be used during the operation to monitor the RLN in real time.

Step 7: sinking the thyroid
After the lower part of the thyroid gland was completely free, the retractor was adjusted between the SMs and the thyroid gland. Using the retractor’s force and simultaneously pulling down the thyroid gland, an ultrasonic scalpel was used to operate in the gap between the SMs and the thyroid gland. The disconnection of the lower pole of bilateral central lymph nodes to the upper edge of the unnamed artery was performed. Finally, the bilateral lobes and isthmus of the thyroid gland and the lymph nodes in the left central region and VIa region were completely removed to achieve the effect of radical tumor treatment (Figure 9). The chief surgeon should be familiar with the above seven steps, and undergoes training to standardize the procedures of the operation and reduce the occurrence of surgical complications.

Closure: the operation site was flushed with sterile water and checked for bleeding. A drainage tube was placed in the bed of the thyroid gland under the axillary incision to drain blood and fluid. The axillary incision was sutured layer-by-layer. The total duration of the surgery was approximately 2 hours.
Postoperative considerations and tasks
The patient began oral intake of a liquid diet 6 hours after surgery, and the drainage tube was removed 3 days after the operation. Vocal cord function was checked by laryngoscopy the day after surgery. Laryngoscopy was performed before and after the operation, and the results were normal without vocal cord paralysis. It is necessary to observe the patient’s voice after the operation and determine whether there is hoarseness. The common rules of thyroid surgery dictate discharge from the hospital. The patient was discharged the same day the drainage tube was removed. The final pathological findings of the patient were bilateral microscopic papillary carcinoma with left central region lymph node 0/6, 6a region lymph node 0/2, and 6b region lymph node 0/3. To further observe the patient’s recovery after discharge, telephone follow-up was conducted at 1, 3, 6, and 12 months after surgery.
Tips and pearls
The dynamic and progressively repositioned retractor is very important in our surgery. Our modified posterior approach uses a retractor to lift the muscle and thyroid together with the central lymph nodes, which can not only solve the problem of the visual blind area but also provide good tension and free-up hands for operation.
Discussion
This video shows in detail all steps necessary to perform an axillary approach for total thyroidectomy with the posterior approach. We also provide a schematic diagram to help everyone understand the position of the retractor during surgical operations and the relationship between various important structures (Figures 3-9).
In addition to the transaxillary approach, there are other approaches to complete endoscopic thyroidectomy, including unilateral axilla-bilateral areola (UABA) with gas or transoral endoscopic thyroidectomy vestibular approach (TOETVA), which are commonly used. Zhang et al. reported that the incidence of postoperative transient hypoparathyroidism and postoperative permanent hypoparathyroidism were 17.9% (27/151) and 2.0% (3/151) after total thyroidectomy and central lymph node dissection via UABA, lower than that in the open group (P<0.05 respectively). The rates in the open surgery group were 29.7% (44/148) and 6.8% (10/148) (9). Park et al. reported that endoscopic total thyroidectomy through UABA is technically feasible and has comparable surgical completeness to open total thyroidectomy for papillary microcarcinoma within 1 cm (10). Meanwhile, many articles have studied the safety and surgical completeness of TOETVA with conventional open thyroidectomy (COT) in patients undergoing total thyroidectomy and central lymph node dissection. Sun et al. reported that TOETVA is a safe approach in selected patients with papillary thyroid carcinoma and exhibits similar efficacy to COT in terms of surgical success (11). Li et al. reported that TOETVA is a safe and feasible technique for better cosmetic effects and with similar surgical outcomes compared to conventional open surgery for the studied patients that required total thyroidectomy (12).
Previously, axillary surgery, chest/breast surgery, and oral thyroid surgery were often performed from the front of the thyroid gland, similar to open surgery, requiring one hand to pull the thyroid gland for surgery. For this reason, it is difficult to complete contralateral thyroidectomy using traditional endoscopic instruments (13). Some studies have reported robot-assisted total thyroidectomy through a unilateral axillary approach (14-18). Unlike the end of the endoscopic instrument, robotic arms can be steered freely and make removal of the contralateral lobe of the thyroid easily. However, robotic surgery is far more expensive than traditional laparoscopic surgery and is not universally available (19). As part of our improved procedures, we use a retractor to lift the thyroid and central lymph node tissue as a whole, which frees one hand, provides better tension, and thoroughly removes the central lymph nodes. The adjustment of the hook is crucial throughout the entire surgical process. Each sinking step requires adjustment of the retractor to help provide tension. In our schematic diagram, we can clearly see the position of the retractor and its relationship with surrounding tissues. Through the posterior approach and the operation steps of the seven-sinking method, total thyroidectomy and bilateral central lymph node dissection can be achieved relatively easily. Compared with other approaches, the axillary approach still has certain difficulties and limitations in bilateral thyroid surgery, mainly due to the obstruction of the trachea. If the trachea is hard to sink, it is difficult for the lens and instruments to operate on the opposite side, resulting in certain surgical difficulty.
Given our technique’s reliance on retractions with the retractor, excessive traction may cause RLN iatrogenic damage. Continuous IONM (C-IONM) seems to be superior to intermitted IONM (I-IONM) because it enhances standardization by permanent VN stimulation, and it provides entire and constant RLN function monitoring when the surgeon adjusts the retractor (20). Zhang et al. reported their clinical experience of the use of percutaneous continuous neuromonitoring in robotic bilateral axilla-breast thyroid surgery (21). C-IONM provides a simplification of the continuous monitoring procedure in thyroid surgery.
Our concept for lymph node dissection in the central region is to perform a final dissection of adipose lymph node tissue with the thyroid. After lifting the thyroid gland with a retractor, the thyroid gland is used to pull the adipose lymph node tissue, thereby thoroughly removing the adipose lymph node tissue and avoiding tissue retraction. Lymph node dissection is routinely performed in Nanfang Hospital of Southern Medical University (22). In previous articles from Nanfang Hospital of Southern Medical University, 521 patients underwent unilateral lobectomy and lymph node dissection. The mean numbers of lymph nodes retrieved and positive lymph nodes were 5.7±4.3 (range, 1–30) and 1.0±1.8 (range, 0–12), respectively (6). Our study enrolled 48 consecutive patients who underwent RGTETT with the posterior approach. The mean numbers of lymph nodes retrieved and positive lymph nodes was 10.7 (range, 5–17) and 1.23 (range, 0–6), respectively.
Limitations
The number of cases of bilateral endoscopic surgery in Nanfang Hospital of Southern Medical University was not very large. These cases are not enrolled in a prospective study. This paper explains how the posterior approach can partially solve the drawbacks of traditional endoscopic surgery and promotes the development of this technology. We would collect data from more patients to analyse the outcomes (surgical, pathological, oncological, and cosmetic) in the future.
Conclusions
This video and schematic diagram support the fact that this posterior approach could be a viable alternative to open surgery in well-selected patients and will be useful to all surgeons who are interested in posterior approaches for total thyroidectomy.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-204/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-204/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-204/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the ethics committee of Nanfang Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol 2020;16:17-29. [Crossref] [PubMed]
- Cong R, Li X, Ouyang H, et al. Gasless, endoscopic trans-axillary thyroid surgery: our series of the first 51 human cases. World J Surg Oncol 2022;20:9. [Crossref] [PubMed]
- Li T, Zhang Z, Chen W, et al. Comparison of quality of life and cosmetic result between open and transaxillary endoscopic thyroid lobectomy for papillary thyroid microcarcinoma survivors: A single-center prospective cohort study. Cancer Med 2022;11:4146-56. [Crossref] [PubMed]
- Rossi L, Materazzi G, Bakkar S, et al. Recent Trends in Surgical Approach to Thyroid Cancer. Front Endocrinol (Lausanne) 2021;12:699805. [Crossref] [PubMed]
- Zhou Y, Cai Y, Sun R, et al. Gasless transaxillary endoscopic thyroidectomy for unilateral low-risk thyroid cancer: Li's six-step method. Gland Surg 2021;10:1756-66. [Crossref] [PubMed]
- Ge JN, Yu ST, Sun BH, et al. A novel anatomy-based five-settlement method for endoscopic thyroid lobectomy and ipsilateral central compartment neck dissection via gasless unilateral axillary approach: a preliminary report. Front Endocrinol (Lausanne) 2023;14:1147313. [Crossref] [PubMed]
- Chen W, Yu S, Sun B, et al. The learning curve for gasless transaxillary posterior endoscopic thyroidectomy for thyroid cancer: a cumulative sum analysis. Updates Surg 2023;75:987-94. [Crossref] [PubMed]
- Randolph GW, Dralle H, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Zhang Y, Du J, Ma J, et al. Unilateral axilla-bilateral areola approach for thyroidectomy by da Vinci robot vs. open surgery in thyroid cancer: a retrospective observational study. Gland Surg 2021;10:1291-9. [Crossref] [PubMed]
- Park KN, Jung CH, Mok JO, et al. Prospective comparative study of endoscopic via unilateral axillobreast approach versus open conventional total thyroidectomy in patients with papillary thyroid carcinoma. Surg Endosc 2016;30:3797-801. [Crossref] [PubMed]
- Sun H, Wang X, Zheng G, et al. Comparison Between Transoral Endoscopic Thyroidectomy Vestibular Approach (TOETVA) and Conventional Open Thyroidectomy for Patients Undergoing Total Thyroidectomy and Central Neck Dissection: A Propensity Score-Matching Analysis. Front Oncol 2022;12:856021. [Crossref] [PubMed]
- Li Y, Liu Z, Wang Y, et al. Is transoral endoscopic thyroidectomy safe for total thyroidectomy compared to open thyroidectomy? A propensity-score matched cohort study with papillary thyroid carcinoma. J Surg Oncol 2023;128:502-9. [Crossref] [PubMed]
- Kim EY, Lee KH, Park YL, et al. Single-Incision, Gasless, Endoscopic Trans-Axillary Total Thyroidectomy: A Feasible and Oncologic Safe Surgery in Patients with Papillary Thyroid Carcinoma. J Laparoendosc Adv Surg Tech A 2017;27:1158-64. [Crossref] [PubMed]
- Ikeda Y, Takami H, Sasaki Y, et al. Endoscopic neck surgery by the axillary approach. J Am Coll Surg 2000;191:336-40. [Crossref] [PubMed]
- Ryu CH, Seok J, Jung YS, et al. Novel robot-assisted thyroidectomy by a transaxillary gas-insufflation approach (TAGA): a preliminary report. Gland Surg 2020;9:1267-77. [Crossref] [PubMed]
- Papini P, Fregoli L, Materazzi G. Right transaxillary robotic-assisted total thyroidectomy (with). J Visc Surg 2020;157:353-4. [Crossref] [PubMed]
- Brunaud L, Germain A, Zarnegar R, et al. Robotic thyroid surgery using a gasless transaxillary approach: cosmetic improvement or improved quality of surgical dissection? J Visc Surg 2010;147:e399-402. [Crossref] [PubMed]
- Kim JK, Choi SH, Choi SM, et al. Single-port transaxillary robotic thyroidectomy (START): 200-cases with two-step retraction method. Surg Endosc 2022;36:2688-96. [Crossref] [PubMed]
- Childers CP, Maggard-Gibbons M. Estimation of the Acquisition and Operating Costs for Robotic Surgery. JAMA 2018;320:835-6. [Crossref] [PubMed]
- Dionigi G, Chiang FY, Hui S, et al. Continuous Intraoperative Neuromonitoring (C-IONM) Technique with the Automatic Periodic Stimulating (APS) Accessory for Conventional and Endoscopic Thyroid Surgery. Surg Technol Int 2015;26:101-14. [PubMed]
- Zhang D, Wang C, Wang T, et al. Clinical Experience of Use of Percutaneous Continuous Nervemonitoring in Robotic Bilateral Axillo-Breast Thyroid Surgery. Front Endocrinol (Lausanne) 2021;12:817026. [Crossref] [PubMed]
- Yu ST, Ge JN, Sun BH, et al. Lymph node yield in the initial central neck dissection (CND) associated with the risk of recurrence in papillary thyroid cancer: A reoperative CND cohort study. Oral Oncol 2021;123:105567. [Crossref] [PubMed]


