
Extensive intraductal component as a factor determining local recurrence of breast cancer: a systematic review and meta-analysis
Highlight box
Key findings
• This meta-analysis suggests that breast cancer patients with extensive intraductal component (EIC) positive tumor have higher rates of local recurrence in overall population, but this cannot be generalized to patients who achieve negative margin or receive irradiation.
What is known and what is new?
• EIC is not a contraindication for breast-conserving surgery. The challenging point is how to obtain negative margin for EIC-containing lesions during breast-conserving surgery.
What is the implication, and what should change now?
• To improve treatment outcomes, accurate predictive tools for EIC-positive tumor, precise estimation in the extent of EIC and proper localization techniques remain the issues for further studies.
Introduction
Multimodality treatment is essential for the improvement in breast cancer survival, however, surgery remains the mainstay option in early breast cancer patients. Multiple prospective randomized trials have demonstrated that breast-conserving therapy and mastectomy showed no significant difference in disease-free survival, distant disease-free survival, and overall survival (1-3). Ipsilateral breast tumor recurrence (IBTR) correlated with systemic recurrence in several studies but the impact of IBTR on overall survival was still controversial. Several studies demonstrated that IBTR cannot translate into decreased survival, but it resulted in a higher risk for distant metastases. Many researchers hypothesized that IBTR might be the cause of systemic recurrence, however, others suggested that IBTR was an indicator of aggressive disease coexisting with micrometastasis (4-6). Thus, locoregional control remained an important goal of treatment for early-stage breast carcinoma. An increased risk of local recurrence was associated with younger patients, involved resection margin, non-luminal biological subtype, lymphovascular invasion (LVI), and an extensive intraductal component (EIC) (5,7,8).
EIC is defined as tumors that are composed of ductal carcinoma in situ (DCIS) component comprising at least 25% and extending to surrounding normal breast tissue. Many studies suggested that the presence of EIC is associated with the amount of residual disease after breast-conserving surgery. However, the correlation of EIC and local recurrence was unclear (9-11). A study showed that local recurrence rates for EIC-negative tumors and EIC-positive tumors were 2% to 9% and 10% to 30%, respectively (12). Nevertheless, further studies have been reported that there was no association between EIC and local recurrence when resection margin is negative (10,13,14). Additionally, there were neither any systematic reviews nor controlled trials that demonstrated the relationship between EIC and local failure. The purpose of this study is to determine the effect of EIC on local recurrence risk, focusing on margin and adjuvant radiation status. We present this article in accordance with the MOOSE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-137/rc).
Methods
Study sources and searches
A systematic literature search was performed using PubMed and Scopus databases. All relevant studies were published from the inception to December 31st, 2020. The Medial Subject Heading (MESH) terms “Extensive intraductal component”, and “Breast” were used. The search was conducted in August 2021. Search strategy was shown in Appendix 1.
Our inclusion criteria were as follows: (I) breast cancer with EIC as a factor of local recurrence; (II) local recurrence was reported as a major focus.
Studies were excluded on the basis of following criteria: (I) reviews, meta-analyses, abstracts, letters, case report, case series, commentaries, or duplicated publications; (II) overlapping articles; (III) unavailable local recurrence rate; (IV) studies of other cancers and (V) non-English published studies.
The primary objective of this study was to report the local recurrence rate in EIC-positive patients comparing to EIC-negative patients. We focused on the patients who received breast-conserving surgery, however, there was one study that included only mastectomy patients. Prespecified subgroup analysis included margin status in pathological report and adjuvant radiation, either whole breast irradiation or any partial breast irradiation.
Data extraction and quality assessment
Two researchers (Polchai N and Thongvitokomarn S) independently reviewed titles and abstracts of all studies. Disagreement on each study was solved by discussion between two authors. Next, they screened full-text articles for inclusion. Data from eligible studies were extracted and checked by two researchers. For each study, the following data were considered: first author, year of publication, sample size, local recurrence rate, EIC status, adjuvant radiation and margin status of the specimen. The primary outcome of the meta-analysis was a local recurrence rate in EIC-positive patients compared with EIC-negative patients. The following results allowed for meta-analysis: (I) local recurrence in all studies; (II) local recurrence in margin negative studies and received any type of irradiation; and (III) local recurrence in margin negative studies and received whole breast irradiation. All discrepancies were discussed and resolved by consensus of the researchers.
According to non-randomized studies, the quality of all studies was evaluated with the Newcastle Ottawa Scale (15) (Tables S1,S2).
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were used to estimate the effect of categorical outcomes. If such data were not provided, we had to calculate these. Meta-analysis of each outcome was analyzed by RevMan 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and forest plots were used to present the analysis results. The statistical heterogeneity among studies was calculated using I2 statistics and P values. The heterogeneity was considered significant where I2>50% or P<0.05. The Inverse Variance fixed effects and random effects model were used to analyze the OR in each study. Publication bias of all included studies was exhibited in funnel plot. Sensitivity analyses were conducted by using an alternative meta-analysis model and by excluding studies which included mastectomy. Additionally, we performed subgroup analysis to investigate the heterogeneity.
Results
We initially identified 346 potentially eligible studies from PubMed and 393 studies from Scopus. Duplicated studies were removed and a total of 434 studies were included for abstract screening. Of these relevant studies, 378 were excluded basing on their titles or abstracts, i.e., not report local recurrence rate, not report EIC as a factor of local recurrence or not in English language.
Among the remaining 56 studies which could be retrieved in full text, 24 studies were excluded from the following basis: no comparative outcome between EIC positive and EIC negative patients (8 studies); unavailable full text (6 studies); no local recurrence as an outcome (4 studies); publications in languages other than English (4 studies); duplicated studies (2 studies). The PRISMA scheme was shown in Figure 1. All included studies were observational studies and classified as low to intermediate risk of bias according to Newcastle Ottawa Scale (Tables S1,S2).
Consequently, there were 32 studies for data extraction and meta-analysis, comprising 4,290 and 15,143 patients in the EIC-positive and EIC-negative groups respectively. A total of 12 studies demonstrated the margin status (14,16-26) but the others did not present this information (8,10,27-44). Among these 32 studies, there were four studies, which reported the local recurrence in margin negative patients who received breast-conserving surgery with any types of irradiation (17,21,24,26). Three out of four studies reported the local recurrence in margin negative patients and received adjuvant whole breast irradiation (17,21,24). Irradiation treatments in those four studies included whole breast irradiation (two studies), whole breast and intra-operative irradiation (one study) and intra-operative irradiation only (one study). Baseline characteristic of all 32 studies was summarized in Table 1.
Table 1
| First author | Year | Follow-up (months), median (range)/median/mean (range) | N | Adjuvant radiation | Report margin status | Negative margin | Definition of EIC | Type of local surgery | Remarks | |
|---|---|---|---|---|---|---|---|---|---|---|
| BCS | Mastectomy | |||||||||
| Ni (27) | 2014 | 75 (0.23–133.8) | 633 | n/a | n/a | n/a | n/a | n/a | ||
| Jacquemier (16) | 1990 | 71 (n/a) | 496 | Yes | Yes | 98.4% | >25% | 100% | ||
| Elsayed (28) | 2016 | 60 (12–120) | 238 | Yes | n/a | n/a | n/a | 100% | ||
| Krishnan (17) | 1992 | 58 (14–110) | 245 | Yes | Yes | 100% | >25% | 100% | ||
| Leborgne (18) | 1995 | 75 (31–248) | 817 | Yes | Yes | 97% | >25% | 100% | ||
| Perez (19) | 2003 | 79 (48–360) | 1,345 | Yes | Yes | 71.4% | >25% | 100% | ||
| Salvadori (20) | 1997 | 133.5 (n/a) | 2,189 | Yes | Yes | 95.4% | >25% | 100% | ||
| Zafrani (29) | 1989 | 103 (52–301) | 424 | Yes | n/a | n/a | >25% | 100% | ||
| Voogd (30) | 2001 | 117 (n/a) | 660 | Yes | n/a | n/a | >10% | 100% | ||
| Boyages (31) | 1990 | 80 (50–202) | 600 | Yes | n/a | n/a | >25% | 100% | ||
| Leong (21) | 2004 | 80 (n/a) | 452 | Yes | Yes | 77.8% | n/a | 100% | Report negative margin | |
| Galper (32) | 1999 | 125 (3–217) | 383 | Yes | n/a | n/a | n/a | 100% | ||
| Voogd (22) | 1999 | n/a | 834 | Yes | Yes | 48.4% | 10 ducts involvement | 100% | ||
| Tenea-Cojan (33) | 2016 | 120 (n/a) | 303 | n/a | n/a | n/a | n/a | 100% | ||
| Osteen (34) | 1987 | 60 (n/a) | 300 | Yes | n/a | n/a | >25% | 100% | ||
| Hurd (10) | 1997 | 84 (48–180) | 133 | Yes | n/a | n/a | >25% | 100% | ||
| Fodor (35) | 2000 | 120 (11–120) | 415 | No | n/a | n/a | >25% | 100% | ||
| Recht (36) | 1988 | 63 (3–181) | 597 | Yes | n/a | n/a | >25% | 100% | ||
| Schnitt (37) | 1989 | 75 (49–183) | 515 | Yes | n/a | n/a | >25% | 100% | ||
| Touboul (38) | 1999 | 47 (6–217) | 528 | Yes | n/a | n/a | >25% | 100% | ||
| Veronesi (8) | 1995 | 102 (n/a) | 1,637 | Yes | n/a | n/a | n/a | 100% | ||
| Cannon (23) | 2013 | 61 (n/a) | 277 | Yes | Yes | 94% | n/a | 100% | ||
| Lai (39) | 2016 | 82.8 (n/a) | 293 | No | n/a | n/a | n/a | 0% | 100% | |
| Ha (40) | 2019 | 79.9 (7–133) | 6,136 | n/a | n/a | n/a | >25% | 70% | ||
| Gage (24) | 1996 | 109 (n/a) | 340 | Yes | Yes | 61.4% | n/a | 100% | Report negative margin | |
| Paterson (25) | 1992 | 44.4 (24–84) | 236 | Yes | Yes | 40% | >25% | 100% | ||
| Eberlein (41) | 1990 | 91 (51–212) | 783 | Yes | n/a | n/a | >25% | 100% | ||
| Kim (42) | 2011 | 61 (6–144) | 762 | n/a | n/a | n/a | n/a | 60% | ||
| Schnitt (14) | 1994 | n/a | 157 | Yes | Yes | 60% | n/a | 100% | ||
| Leopold (43) | 1989 | 75 (4–192) | 516 | Yes | n/a | n/a | >25% | 100% | ||
| Kurtz (44) | 1990 | 71 (n/a) | 496 | Yes (90.30%) | n/a | n/a | >25% | 100% | ||
| Beitsch (26) | 2012 | 60 (0–109) | 1,449 | Yes | Yes | 100% | n/a | 100% | ||
EIC, extensive intraductal component; BCS, breast-conserving surgery; n/a, not applicable.
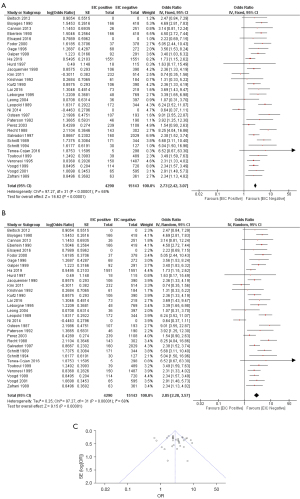
For meta-analysis, local recurrence rate in EIC-positive and EIC-negative groups were analyzed. From all 32 studies, there was a statistically significant difference in local recurrence between EIC-positive patients and EIC-negative patients with respect to both fixed effects model (OR =2.73; 95% CI: 2.42–3.07) and random effects model (OR =2.85; 95% CI: 2.28–3.57). There was a heterogeneity among these 32 studies (I2: 68%; P<0.00001) (Figure 2).
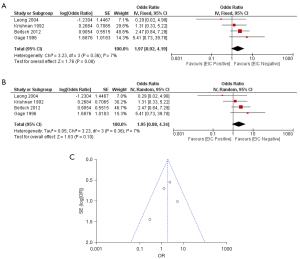
Regarding the margin status, the meta-analysis was done in patients who had negative margin status. From the four studies which reported local recurrence rate in patients who had negative margin, all patients received irradiation. Meta-analysis in these four studies demonstrated a non-statistically significant difference in local recurrence between EIC-positive patients and EIC-negative patients with respect to both fixed effects model (OR =1.97; 95% CI: 0.92–4.19) and random effects model (OR =1.95; 95% CI: 0.88–4.34). There was not a heterogeneity among these four studies (I2: 7%; P=0.36) (Figure 3).
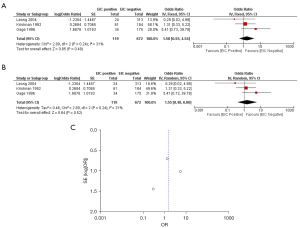
Turning to the margin status and whole breast irradiation, the meta-analysis was done in patients who had negative margin status and received adjuvant whole breast irradiation. Meta-analysis in these three studies demonstrated a non-statistically significant difference in local recurrence between EIC-positive patients and EIC-negative patients with respect to both fixed effects model (OR =1.58; 95% CI: 0.55–4.54) and random effects model (OR =1.55; 95% CI: 0.40–6.00). There was not a heterogeneity among these three studies (I2: 31%; P=0.24) (Figure 4).
In terms of the type of operation, there was one study which included only mastectomy patients (39). Additionally, there were two studies which included both breast-conserving surgery and mastectomy (40,42). However, 60% and 70% of patients from these two studies underwent breast-conserving surgery. Furthermore, there was one study which did not report the type of operation (27).
The meta-analysis was performed with the exclusion of those four studies. There was still a statistically significant difference in local recurrence between EIC-positive and EIC-negative patients [fixed effects model (OR =3.20; 95% CI: 2.81–3.64) and random effects model (OR =3.27; 95% CI: 2.72–3.93), heterogeneity (I2: 44%; P<0.00001)].
Additionally, the meta-analysis was performed with the exclusion of the study that included only mastectomy. Again, there was still a statistically significant difference in local recurrence between EIC-positive patients and EIC-negative patients [fixed effects model (OR =2.71; 95% CI: 2.41–3.06) and random effects model (OR =2.83; 95% CI: 2.25–3.56), heterogeneity (I2: 69%; P<0.00001)].
Discussion
An effective treatment strategy for breast cancer requires both the optimal local control and appropriate systemic therapies. One of the surveillance goals in early-stage breast cancer, which is mostly operated by breast-conserving surgery, is the local recurrence. Positive resection margin was strongly considered as a risk factor for increased local relapse (45), and the presence of EIC seemed to be an additional risk for local relapse apart from negative margin. Thus, we gathered result from current evidence and performed systematic review with meta-analysis to evaluate the impact of EIC on local recurrence
EIC was approximately found in 24–35% of breast cancer patients (46-48). Many studies reported that EIC was a predictor for residual disease after breast-conserving surgery. EIC-positive primary tumors had 38–100% probability of residual disease at re-excision specimens compared to 10–50% probability of carcinoma in the remainder breast in EIC-negative tumors (13,48). Despite the fact that the presence of EIC associated with residual cancer, risk of local recurrence in EIC positive tumor was controversial. Several studies showed that higher local failure rate was higher in patients with EIC (16,44,48). The studies from Kurtz et al. and Freedman et al. found that breast cancer with EIC increased recurrence rates regardless of margin status (44,49). Conversely, further studies demonstrated that IBTR rates in the presence of EIC were not greater if negative margins were achieved (10,14). Moreover, the other study showed that recurrence rates in EIC-positive tumor ranged from 21% to 66% in those with close or positive margin and remarkably decreased to 0% to 6% in those with negative margins (12). As aforementioned data, the presence of EIC was currently not a contraindication for breast-conserving therapy with satisfied margin status.
This study is the first meta-analysis which aimed to define the magnitude of EIC impacted on local recurrence of breast cancer. In our study, local recurrence of breast cancer in EIC-positive and EIC-negative tumor was analyzed from all retrospective 32 studies with follow-up period ranged from 44.4 to 133.5 months. The result demonstrated that EIC-positive patients have significantly higher local recurrence rate than EIC-negative patients according to both fixed and random effect models. However, a heterogeneity was observed among these data, hence we analyzed additional factors that might be specific in increased risk of local recurrence for EIC-positive patients.
Considering margin status, our study demonstrated no statistical difference in local recurrence between EIC-negative and EIC-positive groups who had negative resection margin. Additionally, regarding the studies that reported local relapse in patients who had negative margin and received adjuvant whole breast irradiation, the meta-analysis also showed no statistical difference in local recurrence between EIC-negative and EIC-positive group. A heterogeneity was not detected among these studies. Thus, we could imply that the presence of an EIC did not affect local recurrence of breast cancer if breast-conserving therapy with negative margin can be achieved. Our results were concordant with Society of Surgical Oncology (SSO) and American Society for Radiation Oncology (ASTRO) consensus which recommended no ink on tumor margin was adequate for decrease the rate of IBTR in patients with unfavorable biology, lobular carcinoma or EIC-positive tumors (50). Due to this result, the approaches for obtaining negative margin in EIC-positive patients are necessary and should be recognized in both preoperative and intraoperative assessment.
In terms of preoperative assessment, awareness of EIC-containing lesions is important, and these could be discovered from breast imaging and tissue biopsy. Imaging modalities, which could predict EIC-positive lesions, may guide the extent of resection. Many studies demonstrated that 57.9–65% of lesions, presented with the calcification on mammography with or without a mass, were associated with an EIC, especially when the calcification was greater than 3 cm. Van Goethem et al. showed that intraductal spreading in EIC positive carcinoma was predicted 68%, 48.5% and 34.2% by magnetic resonance (MR), mammogram and ultrasound, respectively and pattern of ductal spreading in EIC containing tumors mostly presented as ductal or linear enhancement around a mass from MR. Moreover, a wider excision after MR gained benefit for 86% of patients with invasive carcinoma and EIC (47). Therefore, MR combined with mammogram and ultrasound should be considered for suspicious EIC-coexisting tumors to make the precise surgical planning.
An essential role of core needle biopsy (CNB) is to identify the probability of EIC in surgical specimens. Several retrospective studies suggested that identification of DCIS with invasive carcinoma on CNB significantly increased the risk of EIC in surgical specimens especially core biopsy tissue comprising more than 45% of the DCIS (51-53). Consequently, this knowledge of DCIS with invasive carcinoma from CNB tissue might facilitate surgeons to increase awareness of EIC during breast-conserving surgery.
Turning to intraoperative period, margin assessment is a critical step to decrease positive margin events. Various types of intraoperative margin assessment including gross evaluation only or microscopic evaluation of the margins have been used, but there were no randomized trials evaluating their efficacy. A meta-analysis of 35 studies demonstrated that frozen section and cytology have the greatest diagnostic accuracy. The sensitivity and specificity were 86% and 96% in frozen section while cytology provided 91% sensitivity and 95% specificity (54). However, these methods have not been generally adopted because they were resource intensive and time consuming. Specimen mammography seemed to be more accessible in some areas. Urano et al. showed that digital breast tomosynthesis (DBT) was superior to digital mammography (DM) in terms of EIC detection from breast specimens. In anteroposterior view, 55% and 65% of EIC positive tumors were detected by DM and DBT, respectively (55).
There were several limitations in our study. First, all included studies were retrospective studies which may lead to selection bias. Second, most of the studies did not clarify margin status as well as definition of EIC, resulting in small number of populations to analyze in subgroup analysis. Third, although this study focused on the margin status, adjuvant radiation and type of surgery, the wide range of study periods (1987–2014) might have influenced the efficacy of surgery, radiotherapy and systemic treatment which brought about a great impact on breast cancer recurrence.
Conclusions
This meta-analysis emphasizes that the presence of an EIC significantly relates to increased risk of local recurrence in breast cancer patients, but this cannot be applied in patients who obtain negative resection margin or receive postoperative irradiation, which are currently the standard treatment. To achieve a clear negative margin, accurately predictive tools for EIC-positive lesions, precise estimation in the extent of EIC and suitable localization techniques for tumor with EIC remain the challenging issues. Further studies in these aspects are required to improve treatment outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-137/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-137/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-137/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Blichert-Toft M, Nielsen M, Düring M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008;47:672-81. [Crossref] [PubMed]
- Fisher B, Anderson S, Fisher ER, et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet 1991;338:327-31. [Crossref] [PubMed]
- Botteri E, Bagnardi V, Rotmensz N, et al. Analysis of local and regional recurrences in breast cancer after conservative surgery. Ann Oncol 2010;21:723-8. [Crossref] [PubMed]
- Meric F, Mirza NQ, Vlastos G, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer 2003;97:926-33. [Crossref] [PubMed]
- Komoike Y, Akiyama F, Iino Y, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer 2006;106:35-41. [Crossref] [PubMed]
- Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 1995;87:19-27. [Crossref] [PubMed]
- Alrahbi S, Chan PM, Ho BC, et al. Extent of margin involvement, lymphovascular invasion, and extensive intraductal component predict for residual disease after wide local excision for breast cancer. Clin Breast Cancer 2015;15:219-26. [Crossref] [PubMed]
- Hurd TC, Sneige N, Allen PK, et al. Impact of extensive intraductal component on recurrence and survival in patients with stage I or II breast cancer treated with breast conservation therapy. Ann Surg Oncol 1997;4:119-24. [Crossref] [PubMed]
- Cedolini C, Bertozzi S, Londero AP, et al. Impact of the presence and quantity of ductal carcinoma in situ component on the outcome of invasive breast cancer. Int J Clin Exp Pathol 2015;8:13304-13. [PubMed]
- Horst KC, Smitt MC, Goffinet DR, et al. Predictors of local recurrence after breast-conservation therapy. Clin Breast Cancer 2005;5:425-38. [Crossref] [PubMed]
- Smitt MC, Nowels K, Carlson RW, et al. Predictors of reexcision findings and recurrence after breast conservation. Int J Radiat Oncol Biol Phys 2003;57:979-85. [Crossref] [PubMed]
- Schnitt SJ, Abner A, Gelman R, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer 1994;74:1746-51. [Crossref] [PubMed]
- Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2020. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Jacquemier J, Kurtz JM, Amalric R, et al. An assessment of extensive intraductal component as a risk factor for local recurrence after breast-conserving therapy. Br J Cancer 1990;61:873-6. [Crossref] [PubMed]
- Krishnan L, Jewell WR, Krishnan EC, et al. Breast cancer with extensive intraductal component: treatment with immediate interstitial boost irradiation. Radiology 1992;183:273-6. [Crossref] [PubMed]
- Leborgne F, Leborgne JH, Ortega B, et al. Breast conservation treatment of early stage breast cancer: patterns of failure. Int J Radiat Oncol Biol Phys 1995;31:765-75. [Crossref] [PubMed]
- Perez CA. Conservation therapy in T1-T2 breast cancer: past, current issues, and future challenges and opportunities. Cancer J 2003;9:442-53. [Crossref] [PubMed]
- Salvadori B, Biganzoli E, Veronesi P, et al. Conservative surgery for infiltrating lobular breast carcinoma. Br J Surg 1997;84:106-9. [PubMed]
- Leong C, Boyages J, Jayasinghe UW, et al. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer 2004;100:1823-32. [Crossref] [PubMed]
- Voogd AC, Peterse JL, Crommelin MA, et al. Histological determinants for different types of local recurrence after breast-conserving therapy of invasive breast cancer. Dutch Study Group on local Recurrence after Breast Conservation (BORST). Eur J Cancer 1999;35:1828-37. [Crossref] [PubMed]
- Cannon DM, McHaffie DR, Patel RR, et al. Locoregional recurrence following accelerated partial breast irradiation for early-stage invasive breast cancer: significance of estrogen receptor status and other pathological variables. Ann Surg Oncol 2013;20:3446-52. [Crossref] [PubMed]
- Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer 1996;78:1921-8. [Crossref] [PubMed]
- Paterson DA, Anderson TJ, Jack WJ, et al. Pathological features predictive of local recurrence after management by conservation of invasive breast cancer: importance of non-invasive carcinoma. Radiother Oncol 1992;25:176-80. [Crossref] [PubMed]
- Beitsch PD, Wilkinson JB, Vicini FA, et al. Tumor bed control with balloon-based accelerated partial breast irradiation: incidence of true recurrences versus elsewhere failures in the American Society of Breast Surgery MammoSite(®) Registry Trial. Ann Surg Oncol 2012;19:3165-70. [Crossref] [PubMed]
- Ni YB, Tsang JY, Chan SK, et al. A novel morphologic-molecular recurrence predictive model refines traditional prognostic tools for invasive breast carcinoma. Ann Surg Oncol 2014;21:2928-33. [Crossref] [PubMed]
- Elsayed M, Alhussini M, Basha A, et al. Analysis of loco-regional and distant recurrences in breast cancer after conservative surgery. World J Surg Oncol 2016;14:144. [Crossref] [PubMed]
- Zafrani B, Vielh P, Fourquet A, et al. Conservative treatment of early breast cancer: prognostic value of the ductal in situ component and other pathological variables on local control and survival. Long-term results. Eur J Cancer Clin Oncol 1989;25:1645-50. [Crossref] [PubMed]
- Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol 2001;19:1688-97. [Crossref] [PubMed]
- Boyages J, Recht A, Connolly JL, et al. Early breast cancer: predictors of breast recurrence for patients treated with conservative surgery and radiation therapy. Radiother Oncol 1990;19:29-41. [Crossref] [PubMed]
- Galper S, Recht A, Silver B, et al. Factors associated with regional nodal failure in patients with early stage breast cancer with 0-3 positive axillary nodes following tangential irradiation alone. Int J Radiat Oncol Biol Phys 1999;45:1157-66. [Crossref] [PubMed]
- Tenea-Cojan TS, Georgescu CV, Corici OM, et al. Histopathological Study on Conservatively Operated Breast Carcinomas. Curr Health Sci J 2016;42:269-82. [PubMed]
- Osteen RT, Connolly JL, Recht A, et al. Identification of patients at high risk for local recurrence after conservative surgery and radiation therapy for stage I or II breast cancer. Arch Surg 1987;122:1248-52. [Crossref] [PubMed]
- Fodor J, Major T, Polgár C, et al. The impact of radiotherapy on the incidence and time of occurrence of local recurrence in early-stage breast cancer after breast conserving therapy. Neoplasma 2000;47:181-6. [PubMed]
- Recht A, Connolly JL, Schnitt SJ, et al. The effect of young age on tumor recurrence in the treated breast after conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 1988;14:3-10. [Crossref] [PubMed]
- Schnitt SJ, Connolly JL, Recht A, et al. Influence of infiltrating lobular histology on local tumor control in breast cancer patients treated with conservative surgery and radiotherapy. Cancer 1989;64:448-54. [Crossref] [PubMed]
- Touboul E, Buffat L, Belkacémi Y, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys 1999;43:25-38. [Crossref] [PubMed]
- Lai SF, Chen YH, Kuo WH, et al. Locoregional Recurrence Risk for Postmastectomy Breast Cancer Patients With T1-2 and One to Three Positive Lymph Nodes Receiving Modern Systemic Treatment Without Radiotherapy. Ann Surg Oncol 2016;23:3860-9. [Crossref] [PubMed]
- Ha SM, Cha JH, Shin HJ, et al. Mammography, US, and MRI to Assess Outcomes of Invasive Breast Cancer with Extensive Intraductal Component: A Matched Cohort Study. Radiology 2019;292:299-308. [Crossref] [PubMed]
- Eberlein TJ, Connolly JL, Schnitt SJ, et al. Predictors of local recurrence following conservative breast surgery and radiation therapy. The influence of tumor size. Arch Surg 1990;125:771-5; discussion 775-7. [Crossref] [PubMed]
- Kim RG, Kim EK, Kim HA, et al. Prognostic significance of molecular subtype in T1N0M0 breast cancer: Korean experience. Eur J Surg Oncol 2011;37:629-34. [Crossref] [PubMed]
- Leopold KA, Recht A, Schnitt SJ, et al. Results of conservative surgery and radiation therapy for multiple synchronous cancers of one breast. Int J Radiat Oncol Biol Phys 1989;16:11-6. [Crossref] [PubMed]
- Kurtz JM, Jacquemier J, Amalric R, et al. Risk factors for breast recurrence in premenopausal and postmenopausal patients with ductal cancers treated by conservation therapy. Cancer 1990;65:1867-78. [Crossref] [PubMed]
- Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010;46:3219-32. [Crossref] [PubMed]
- Stomper PC, Connolly JL. Mammographic features predicting an extensive intraductal component in early-stage infiltrating ductal carcinoma. AJR Am J Roentgenol 1992;158:269-72. [Crossref] [PubMed]
- Van Goethem M, Schelfout K, Kersschot E, et al. MR mammography is useful in the preoperative locoregional staging of breast carcinomas with extensive intraductal component. Eur J Radiol 2007;62:273-82. [Crossref] [PubMed]
- Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol 1990;8:113-8. [Crossref] [PubMed]
- Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys 1999;44:1005-15. [Crossref] [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys 2014;88:553-64. [Crossref] [PubMed]
- Dzierzanowski M, Melville KA, Barnes PJ, et al. Ductal carcinoma in situ in core biopsies containing invasive breast cancer: correlation with extensive intraductal component and lumpectomy margins. J Surg Oncol 2005;90:71-6. [Crossref] [PubMed]
- Barbalaco Neto G, Rossetti C, Fonseca FL, et al. Ductal carcinoma in situ in core needle biopsies and its association with extensive in situ component in the surgical specimen. Int Arch Med 2012;5:19. [Crossref] [PubMed]
- Matsumoto H, Ishii A, Nakada N, et al. Predictive value of ductal carcinoma in situ with invasive breast cancer in core needle biopsies for final pathologic size of intraductal elements. Virchows Arch 2022;480:739-48. [Crossref] [PubMed]
- St John ER, Al-Khudairi R, Ashrafian H, et al. Diagnostic Accuracy of Intraoperative Techniques for Margin Assessment in Breast Cancer Surgery: A Meta-analysis. Ann Surg 2017;265:300-10. [Crossref] [PubMed]
- Urano M, Shiraki N, Kawai T, et al. Digital mammography versus digital breast tomosynthesis for detection of breast cancer in the intraoperative specimen during breast-conserving surgery. Breast Cancer 2016;23:706-11. [Crossref] [PubMed]



