
Study on the application value of fluorescent laparoscopy in pancreatic tumor surgery
Highlight box
Key findings
• By reasonably controlling the administration time and dose of indocyanine green during surgery, some pancreatic tumors can be fluorescently imaged, which is beneficial for intraoperative tumor localization and margin determination.
What is known and what is new?
• Fluorescence laparoscopy is widely used in liver surgery, but rarely used in pancreatic surgery;
• Through this application of fluorescence laparoscopy to pancreatic tumors of different pathological types, we found that pancreatic tumors are capable of fluorescence imaging, and the imaging characteristics of tumors of different pathological types are different.
What is the implication, and what should change now?
• Combined with the results of this study, the use of fluorescent laparoscopy in pancreatic tumors can be increased and deserves further clinical promotion.
Introduction
Pancreatic tumors are one of the common tumors of the gastrointestinal tract, with insidious onset and often no clinical symptoms in the early stage (1). The World Health Organization (WHO) classifies them into three parts: benign epithelial tumors and precursor lesions, malignant epithelial tumors, and neuroendocrine tumors (2). Currently, surgery remains the only curative treatment for pancreatic tumors (3). Although abdominal enhanced thin-layer computed tomography (CT), magnetic resonance imaging (MRI), and the recently emerged 3-dimensional (3D) visualization technology can provide accurate preoperative assessment for patients with pancreatic tumors, there may still be additional findings identified during surgery. In hepatobiliary and pancreatic surgery, fluorescent laparoscopy is a widely applied technique in laparoscopic liver surgery (4), with good clinical application results, providing great assistance to the surgery in terms of identification of the bile leaks (5,6). However, it has not broadly been applied to pancreatic surgery. Currently, attempts at its utilization are in laparoscopic duodenum-preserving pancreatic head resection (LDPPHR), where indocyanine green (ICG) imaging is used to effectively avoid bile duct injury (7-9). However, there are few reports on the study of fluorescent imaging of pancreatic tumors themselves. Moreover, unlike laparoscopic liver surgery, for which there are certain differences in ICG injection time, administrative route, and dosage among various centers (10,11), such parameters are still unclear in pancreatic surgery. However, its use in open pancreatic surgery may be beneficial in tumor localization, margin determination, with the potential to shorten operative time and also avoid excessive resection of pancreatic tissue. The aim of this study was to preliminarily explore the value of fluorescent laparoscopy in the resection of pancreatic tumors and provide guidance for the surgery of patients with pancreatic tumors. We present this article in accordance with the STROBE and AME Case Series reporting checklists (available at https://gs.amegroups.com/article/view/10.21037/gs-23-331/rc).
Methods
Participants
A total of 19 patients diagnosed with pancreatic tumors before surgery from January 2021 to August 2022 in the Department of Hepatobiliary Surgery at the First Affiliated Hospital of Wannan Medical College were selected as the research participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Wannan Medical College (No. 2023-161) and individual consent for this retrospective analysis was waived.
The inclusion criteria were as follows: (I) diagnosis of pancreatic tumor by preoperative enhanced CT, MRI, and other appropriate imaging methods, and with surgical resection indications; (II) 18–80 years old; (III) complete case information available.
The exclusion criteria were as follows: (I) patients with a history of allergy to the ICG formulation or iodine were excluded to prevent anaphylactic shock; (II) patients with a past history of iodine allergy (this preparation contains iodine and therefore has a potential to cause iodine allergy).
Study methods
ICG for injection (25 mg; Dandong Medical Creation, Dandong, China) was used. After injecting ICG for 5–10 seconds during surgery, the fluorescence laparoscope (Nanjing Nuoyuan Fluorescence Lens, Nanjing, China) was set to detection of the luminescent ICG, and tumor tissue was marked as green or purple (different brands of fluorescent laparoscopes display different colors). The fluorescence laparoscope was alternated between white light, fluorescence, and black and white light, to observe and record the imaging characteristics of pancreatic tumors. White light is a normal image seen under laparoscopy; black and white light is the original image under fluorescence laparoscopy; fluorescence is an image processing based on black and white light for observing lesions.
In this study, we used the first window ICG fluorescence imaging (intraoperative intravenous injection of ICG), corresponding second window ICG, which is intravenous injection 24 hours before surgery. The specific method is as follows: dissolve 25 mg in 10 mL of the provided sterile injection water, then dilute with 10 mL of physiological saline, extract 1 mL of the solution, and inject it intravenously. The fluorescence laparoscope can be used to observe the pancreatic tumor fluorescence imaging effect after 1 minute.
Fluorescence effect analysis
The effects of ICG injection time and dosage on tumor imaging were analyzed. Postoperative pathology of each patient’s tumor was characterized and correlated with the intraoperative imaging effects. The fluorescence imaging characteristics of different types of pancreatic tumors were recorded and the etiology of these characteristics were assessed with imaging.
Statistical analysis
The statistical software SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. Count data were expressed as n (%), and inter-group comparisons were made using the Chi-square (χ2) test; measurement data were expressed as , and inter-group comparisons were made using the χ2 test. The test level (α) was 0.05.
Results
General clinical data of patients
A total of 19 patients were included in this study, with 12 males and 7 females, and a median age of 67 years. They underwent 18 laparoscopic surgeries, including 7 laparoscopic pancreaticoduodenectomies (LPD), 5 laparoscopic radical antegrade modular pancreatosplenectomies (LRAMPS; including 1 case combined with celiac trunk resection), 3 laparoscopic central pancreatectomies (LCP), 1 laparoscopic spleen-preserving distal pancreatectomy, 1 laparoscopic local pancreatectomy, and 1 LDPPHR. One open surgery was performed after laparoscopic exploration, which was pancreaticoduodenectomy (PD) combined with superior mesenteric vein (SMV) resection and reconstruction. Postoperative pathology confirmed 12 cases of pancreatic adenocarcinoma, 4 cases of pancreatic cystic tumors, 1 case of neuroendocrine tumor, and 2 cases of inflammatory lesions. For more details, see Table 1.
Table 1
| Patient | Gender | Age (years) | BMI, kg/m2 | Tumor marker (U/mL) | Tumor location | Surgical method | Postoperative pathology |
|---|---|---|---|---|---|---|---|
| 1 | Male | 70 | 20.42 | CA199 >1,200 | Pancreatic body-tail | LRAMPS | Moderately to poorly differentiated adenocarcinoma |
| 2 | Female | 57 | 23.11 | Normal | Pancreatic head-uncinate | LPD | Acinar cell carcinoma |
| 3 | Male | 80 | 24.91 | CA199 >1,200; CA125 81.50 | Pancreatic head | LPD | Moderately differentiated adenocarcinoma |
| 4 | Female | 73 | 26.22 | CA199 268.00 | Pancreatic head | PD (with SMV resection and reconstruction) | Moderately differentiated adenocarcinoma |
| 5 | Female | 72 | 21.64 | Normal | Pancreatic head | LCP | Microcystic serous cystadenoma |
| 6 | Male | 67 | 28.69 | CA199 706.51 | Pancreatic body-tail | LRAMPS | Moderately differentiated adenocarcinoma |
| 7 | Male | 74 | 23.57 | CA199 900.72 | Pancreatic head-uncinate | LPD | Moderately differentiated adenocarcinoma |
| 8 | Male | 51 | 21.30 | CA199 >1,200 | Pancreatic head-uncinate | LPD | Moderately to poorly differentiated adenocarcinoma |
| 9 | Male | 64 | 22.85 | Normal | Pancreatic head | LPD | Inflammatory disease |
| 10 | Male | 45 | 20.20 | Normal | Pancreatic neck | LCP | Neuroendocrine tumor (G1) |
| 11 | Female | 56 | 27.41 | Normal | Pancreatic neck | LCP | Inflammatory disease |
| 12 | Female | 69 | 25.81 | CA199 111.54 | Pancreatic body-tail | LRAMPS (with celiac trunk resection) | Moderately differentiated adenocarcinoma |
| 13 | Male | 69 | 22.66 | CA199 124.85 | Pancreatic head-uncinate | LPD | Moderately to poorly differentiated adenocarcinoma |
| 14 | Female | 63 | 24.03 | Normal | Pancreatic head | LDPPHR | Serous cystadenoma |
| 15 | Male | 48 | 24.28 | Normal | Pancreatic neck | Laparoscopic local resection | Solid pseudopapillary tumor |
| 16 | Male | 73 | 26.89 | CA125 65.30 | Pancreatic body | LRAMPS | Moderately differentiated adenocarcinoma |
| 17 | Male | 73 | 24.27 | CA199 >1,200; CA125 44.70 | Pancreatic head | LPD | Moderately differentiated adenocarcinoma |
| 18 | Female | 35 | 24.68 | Normal | Pancreatic body | LDP (Kimura) | Solid-pseudopapillary tumor |
| 19 | Male | 54 | 24.80 | CA199 647.04 | Pancreatic body | LRAMPS | Moderately differentiated adenocarcinoma |
BMI, body mass index; CA, carbohydrate antigen; LRAMPS, laparoscopic radical antegrade modular pancreatosplenectomy; LPD, laparoscopic pancreaticoduodenectomy; PD, pancreaticoduodenectomy; SMV, superior mesenteric vein; LCP, laparoscopic central pancreatectomy; LDPPHR, laparoscopic duodenum-preserving pancreatic head resection.
Tumor fluorescence imaging
Of the 19 pancreatic tumor patients included in this study, 78.9% (15/19) had tumor fluorescence imaging performed successfully. According to the tumor fluorescence imaging characteristics and postoperative pathology, the following 4 categories were identified: (I) pancreatic cancer, with 83.3% imaging performed successfully (10/12): 8 cases of moderately differentiated adenocarcinoma (2 cases without imaging), 3 cases of moderately to poorly differentiated adenocarcinoma, and 1 case of acinar cell carcinoma; (II) pancreatic cystic tumors, 50% with imaging performed successfully (2/4): serous cystadenomas without imaging, solid pseudopapillary tumors with imaging: 1 case of pancreatic microcystic serous cystadenoma, 1 case of serous cystadenoma, 1 case of solid pseudopapillary tumor, and 1 case of solid-cystic pseudopapillary tumor; (III) 1 case of pancreatic neuroendocrine tumor (pNET), with imaging performed successfully; and (IV) 2 cases of inflammatory lesions, both with imaging performed successfully. For specific tumor imaging details, see Table 2. And, the pictures of a typical procedure are shown in Figures 1-7.
Table 2
| Patient | Pathology type | Imaging status |
|---|---|---|
| 1 | Moderately-poorly differentiated adenocarcinoma | Tumor peritumor imaging |
| 2 | Acinar cell carcinoma | Tumor peritumor imaging |
| 3 | Moderately differentiated adenocarcinoma | Tumor peritumor imaging |
| 4 | Moderately differentiated adenocarcinoma | Tumor peritumor imaging |
| 5 | Microcystic serous cystadenoma | Tumor not imaged |
| 6 | Moderately differentiated adenocarcinoma | Tumor peritumor imaging |
| 7 | Moderately differentiated adenocarcinoma | Tumor not imaged |
| 8 | Moderately-poorly differentiated adenocarcinoma | Tumor peritumor imaging |
| 9 | Inflammatory lesion | Tumor body imaging |
| 10 | Neuroendocrine tumor (G1) | Tumor body imaging |
| 11 | Inflammatory lesion | Tumor peritumor imaging |
| 12 | Moderately differentiated adenocarcinoma | Tumor not imaged |
| 13 | Moderately-poorly differentiated adenocarcinoma | Tumor peritumor imaging |
| 14 | Serous cystadenoma | Tumor not imaged |
| 15 | Solid-pseudopapillary tumor | Tumor body imaging |
| 16 | Moderately differentiated adenocarcinoma | Tumor peritumor imaging |
| 17 | Moderately differentiated adenocarcinoma | Tumor peritumor imaging |
| 18 | Solid-pseudopapillary tumor | Tumor body imaging |
| 19 | Moderately differentiated adenocarcinoma | Tumor peritumor imaging |
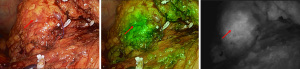

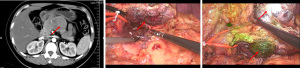
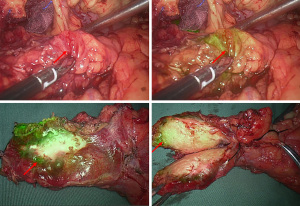
Discussion
Pancreatic surgery is challenging due to its unique anatomical location, which is situated in the retroperitoneum and adjacent to important abdominal vessels such as the superior mesenteric artery (SMA) and SMV (12). The risk of complications from pancreatic surgery is high, with complication rates more than 30% (13). With the increasing popularity of minimally invasive surgeries, pancreatic surgery is also trending towards minimally invasive procedures. Fluorescent technology has been widely and successfully applied in laparoscopic liver resection. Thus, this study aimed to explore the use of fluorescent laparoscopy to guide the surgical resection of pancreatic tumors, improve surgical efficiency, and accelerate the surgical process.
In this study, we injected ICG intravenously during the surgery and found that 83.3% of pancreatic cancers were visualized under fluorescence. In pancreatic cystic tumors, 2 cases of pancreatic serous cystadenomas were not visualized, whereas 2 cases of solid pseudopapillary tumors were visualized. Additionally, 1 case of pNET was visualized and 2 cases of postoperative pathology confirmed as inflammatory lesions (1 preoperative imaging considered cancer and 1 considered cystic tumor) were 100% visualized, with an overall fluorescence visualization rate of 78.9%. In Newton et al.’s study, 11 of 12 pancreatic cancers (91.7%) showed fluorescence, whereas only 3 of 8 benign or low-grade malignant pancreatic tumors [3 intraductal papillary mucinous neoplasms (IPMNs), 2 pNETs, 1 serous cystic neoplasm (SCN), 1 SMA tumor, and 1 nonfunctional (NF) tumor] showed fluorescence (37.5%), specifically 2 IPMNs and 1 SMA (14). Shirata et al. performed ICG fluorescence imaging on 23 pancreatic tumor patients, with 17 achieving effective visualization, and the visualization rate was 74%; the pancreatic cancer visualization rate was 57.1% (4/7). The pancreatic cystic tumor visualization rate was 9.1% (1/11), with solid pseudopapillary tumors not visualized, and the pNET visualization rate was 100% (5/5) (15). This contrasts with our study, where solid pseudopapillary tumors were visualized.
Regarding the choice of ICG injection time, dose, and route, this study used the first window ICG (ICG intravenously injected during surgery). The first window ICG was first studied by Hutteman et al. in 2011, who believed that after intravenous injection of ICG during surgery, there was no clear boundary between the pancreas and the tumor, but the bile duct and choledochojejunostomy could be clearly identified (16). Newton et al.’s study demonstrated that the second window ICG accumulates in pancreatic malignant tumors and can provide real-time feedback during pancreatic surgery. Shirata et al.’s study showed that the first window ICG can visualize pancreatic tumors during surgery. We have previously used the second window ICG (ICG intravenously injected 24 hours before surgery), but the fluorescence visualization of pancreatic tumors was not satisfactory, and some patients experienced allergic reactions and phlebitis (12). At present, most studies on the first or second window ICG are single-center, small-sample studies, and the pathological distribution of pancreatic tumors varies, so there is no definitive conclusion on which is superior.
Furthermore, we found that the fluorescence visualization effect varies for different pathological types of pancreatic tumors. Since ICG is widely distributed throughout the body after intravenous injection, it is selectively and efficiently taken up by hepatocytes and then excreted as free form into bile, entering the intestine through the bile duct and being excreted with feces (17). Therefore, ICG is widely and maturely used in fluorescence imaging technology for liver cancer. Studies have found that the fluorescence imaging characteristics of hepatocellular carcinoma (HCC) tissues with different degrees of differentiation vary. Moderate to well-differentiated HCCs exhibit uniform fluorescence imaging, whereas poorly differentiated HCCs and intrahepatic cholangiocarcinomas exhibit ring-shaped fluorescence imaging. Other benign tumors, such as liver cirrhosis nodules and focal nodular hyperplasia (FNH), are similar to moderate to well-differentiated HCCs. This phenomenon is mainly related to the ability of liver cells to uptake ICG (18). Pancreatic cells cannot uptake ICG like liver cells, and there are very few studies on fluorescence imaging of related pancreatic tumors. In this study, out of 9 patients with pancreatic cancer, 7 showed capsular imaging, whereas 2 did not. Under fluorescent laparoscopy, tumors often appeared as capsular imaging. The possible reason is that the tumor peritumor indicates a higher vascular density than the normal pancreatic tissue capsule. Pancreatic cancer is characterized by its invasiveness and early metastasis, which inevitably depends on the role of tumor angiogenesis (19). At present, there are few studies on the role of neovascularization in the growth and development of pancreatic cancer (20). In this study, we also observed the vascular density of the tumor peritumor tissue and normal pancreatic tissue capsule under a microscope during the surgery, and found that the vascular density of the tumor peritumor tissue was significantly higher than that of the normal capsule tissue (Figure 8). This is in line with our hypothesis, and we plan to continue to conduct further verification. Similarly, capsular imaging of pancreatic cancer was beneficial for intraoperative tumor localization, accelerating the surgical process, and reducing excessive exploration and dissection. Pancreatic serous cystic tumors could not be imaged, whereas pNETs and inflammatory lesions could be fully and clearly imaged. This may also be related to the richer vascular supply around the tumor compared to the surrounding pancreatic parenchyma. However, the samples, types of pancreatic cystic tumors and neuroendocrine tumors included in this study were small, and no effective conclusions can be drawn yet. In 2018, Shirata et al. found that fluorescent laparoscopy could identify 100% of neuroendocrine tumors, and 90.9% of pancreatic cystic tumors could not be imaged. They pointed out that fluorescent laparoscopy could define the tumor range and believe that fluorescence imaging was based on differences in vascular levels.
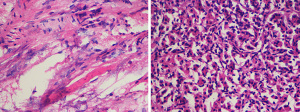
Conclusions
In summary, in this study, we used our center’s ICG administration method to visualize pancreatic tumors, which was found to be beneficial for intraoperative tumor localization. We also conducted a preliminary analysis of the imaging characteristics of different pathological types of tumors, which has certain guiding significance for clinical laparoscopic pancreatic tumor surgery. We plan to continue to increase the sample size and further explore the best methodology fluorescent laparoscopic imaging of pancreatic tumors.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE and AME Case Series reporting checklists. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-331/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-331/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-331/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-331/coif). HC is from Nanjing Nuoyuan Medical Devices Co., Ltd., Nanjing, China. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Wannan Medical College (No. 2023-161) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Strauss J, Heery CR, Schlom J, et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin Cancer Res 2018;24:1287-95. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Halbrook CJ, Lyssiotis CA, Pasca di Magliano M, et al. Pancreatic cancer: Advances and challenges. Cell 2023;186:1729-54. [Crossref] [PubMed]
- Pesce A, Piccolo G, Lecchi F, et al. Fluorescent cholangiography: An up-to-date overview twelve years after the first clinical application. World J Gastroenterol 2021;27:5989-6003. [Crossref] [PubMed]
- Achterberg FB, Sibinga Mulder BG, Meijer RPJ, et al. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann Transl Med 2020;8:1448. [Crossref] [PubMed]
- Zhang P, Luo H, Zhu W, et al. Real-time navigation for laparoscopic hepatectomy using image fusion of preoperative 3D surgical plan and intraoperative indocyanine green fluorescence imaging. Surg Endosc 2020;34:3449-59. [Crossref] [PubMed]
- Hong D, Cheng J, Wu W, et al. How to Perform Total Laparoscopic Duodenum-Preserving Pancreatic Head Resection Safely and Efficiently with Innovative Techniques. Ann Surg Oncol 2021;28:3209-16. [Crossref] [PubMed]
- Cai Y, Zheng Z, Gao P, et al. Laparoscopic duodenum-preserving total pancreatic head resection using real-time indocyanine green fluorescence imaging. Surg Endosc 2021;35:1355-61. [Crossref] [PubMed]
- Chen S, Gao P, Cai H, et al. Indocyanine Green-Enhanced Fluorescence in Laparoscopic Duodenum-Preserving Pancreatic Head Resection: Technique with Video. Ann Surg Oncol 2020;27:3926-7. [Crossref] [PubMed]
- Uchiyama K, Ueno M, Ozawa S, et al. Combined intraoperative use of contrast-enhanced ultrasonography imaging using a sonazoid and fluorescence navigation system with indocyanine green during anatomical hepatectomy. Langenbecks Arch Surg 2011;396:1101-7. [Crossref] [PubMed]
- He K, Hong X, Chi C, et al. Efficacy of Near-Infrared Fluorescence-Guided Hepatectomy for the Detection of Colorectal Liver Metastases: A Randomized Controlled Trial. J Am Coll Surg 2022;234:130-7. [Crossref] [PubMed]
- Ahmad SA, Duong M, Sohal DPS, et al. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg 2020;272:481-6. [Crossref] [PubMed]
- van Dongen JC, Suker M, Versteijne E, et al. Surgical Complications in a Multicenter Randomized Trial Comparing Preoperative Chemoradiotherapy and Immediate Surgery in Patients With Resectable and Borderline Resectable Pancreatic Cancer (PREOPANC Trial). Ann Surg 2022;275:979-84. [Crossref] [PubMed]
- Newton AD, Predina JD, Shin MH, et al. Intraoperative Near-infrared Imaging Can Identify Neoplasms and Aid in Real-time Margin Assessment During Pancreatic Resection. Ann Surg 2019;270:12-20. [Crossref] [PubMed]
- Shirata C, Kawaguchi Y, Kobayashi K, et al. Usefulness of indocyanine green-fluorescence imaging for real-time visualization of pancreas neuroendocrine tumor and cystic neoplasm. J Surg Oncol 2018;118:1012-20. [Crossref] [PubMed]
- Hutteman M, van der Vorst JR, Mieog JS, et al. Near-infrared fluorescence imaging in patients undergoing pancreaticoduodenectomy. Eur Surg Res 2011;47:90-7. [Crossref] [PubMed]
- Kjærgaard K, Frisch K, Sørensen M, et al. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J Hepatol 2021;74:58-65. [Crossref] [PubMed]
- de Graaf W, Häusler S, Heger M, et al. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol 2011;54:738-45. [Crossref] [PubMed]
- Yamaue H, Tsunoda T, Tani M, et al. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study. Cancer Sci 2015;106:883-90. [Crossref] [PubMed]
- Friess H, Langrehr JM, Oettle H, et al. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer 2006;6:285. [Crossref] [PubMed]





