
Tumor size is associated with adjuvant chemotherapy benefit in T1N0M0 triple-negative breast cancer: a multicenter and propensity score matched analysis
Highlight box
Key findings
• Based on a real-world cohort from a large multicenter database and propensity score matched analysis, we found that tumor size was significantly associated with the survival benefit of adjuvant chemotherapy in T1N0 triple-negative breast cancer (TNBC) patients.
What is known and what is new?
• The main prevalent systemic treatment for TNBC is chemotherapy. However, benefit of adjuvant chemotherapy in patients with T1N0M0 TNBC is still unclear, especially for T1a-b patients. There are controversies on recommendations from current guidelines concerning this issue.
• This study evaluated the survival benefit of adjuvant chemotherapy and influential factors in T1N0M0 TNBC patients.
What is the implication, and what should change now?
• Adjuvant chemotherapy significantly improved the breast cancer-free interval only in the T1c population but not in T1a-b patients. Administration of adjuvant chemotherapy in T1a-bN0M0 TNBC patients should be more cautious.
Introduction
Triple-negative breast cancer (TNBC), defined by negative expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), accounts for approximately 10–20% of all breast cancer (BC) cases. It is characterized by more aggressive phonotypes, a higher recurrence rate and relatively poorer outcomes when compared with other BC subtypes (1-3). However, unlike the diverse systemic therapeutic strategies for other BC subtypes, such as hormonal therapy and anti-HER2 targeted therapy, the most common systemic treatment for TNBC is chemotherapy (1,3,4).
With the increased awareness of cancer screening and widespread application of mammography screening, an increasing number of small BCs are being detected (5,6). TNBC is usually symptomatic and rarely detected asymptomatically by mammographic screening due to the rapid growth pattern, but it still accounts for approximately 10–15% of patients diagnosed with small BCs (7,8). Some research showed patients with small BC had a favorable prognosis (6,9-11). However, the benefit of adjuvant chemotherapy in small TNBCs is still unclear. The majority of clinical studies exclude small tumors when exploring the impact of adjuvant chemotherapy on TNBC (12-14). Recently, the KEYNOTE522 study also excluded T1N0 TNBC patients for the evaluation of the benefit of chemotherapy with or without pembrolizumab (15). Although some retrospective studies have discussed the benefit of adjuvant chemotherapy in patients with T1N0M0 TNBC, the results are still controversial (16,17). Therefore, current guidelines have different recommendations for adjuvant chemotherapy on T1N0M0 TNBC. The National Comprehensive Cancer Network (NCCN) guideline recommends no chemotherapy for T1a tumors, considering chemotherapy for T1b tumors if patients have high-risk features and usage of chemotherapy for T1c tumors (18). The St. Gallen guideline recommends that in T1b and T1c tumors, adjuvant chemotherapy should be provided but not routinely for T1a tumors (19). The European Society for Medical Oncology (ESMO) guideline recommends chemotherapy for T1 patients, with the possible exception of very early (T1aN0) tumors (20). There are controversies on the use of adjuvant chemotherapy in T1N0M0 TNBCs, especially in T1a-b patients. In this study, we included T1N0M0 TNBC patients from multiple centers and used propensity score matched (PSM) analysis to evaluate adjuvant chemotherapy benefit in these patients, especially for those with T1a-b tumors. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-189/rc).
Methods
Data source
The study was approved by the independent Ethical Committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (No. 2020-0309). Informed consent was waived by the ethics committee due to its retrospective nature. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study population was retrospectively included from the Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB), which includes more than 70,000 BC cases from 40 medical centers in China. Eligible patients were invasive non-metastatic TNBC diagnosed with tumor size ≤2 cm (T1) and with no axillary lymph node involvement (N0) between January 2009 and December 2021. Patients with the following criteria were excluded: undergoing surgery for in situ carcinoma, receiving neoadjuvant treatment, or bilateral BC. The collected data included patients’ clinicopathological characteristics (age, menopausal status, tumor size, pathological type, tumor grade, ER, PR, HER2, and Ki67) and details of treatment (breast surgery, radiotherapy, chemotherapy). Clinical BC tumor-node-metastasis (TNM) staging was based on the 8th American Joint Committee on Cancer (AJCC) TNM staging manual (21).
Follow-up
All patients were followed up by outpatient visits or calls every 3 months for the first 2 years after surgery, every 6 months between the 3rd and 5th years, and then annually every year until death. The breast cancer-free interval (BCFI) was defined as the length of time from surgery to the first occurrence of the following events: locoregional recurrence of any invasive disease, contralateral invasive BC, distant recurrence, and BC-related death. Overall survival (OS) was defined as the length of time from surgery to any cause of death.
Statistical analysis
Statistical analysis and image construction were performed using IBM SPSS version 25 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism version 8.0 (GraphPad Software, CA, USA), and a two-sided P value of <0.05 was considered statistically significant. The Chi-squared test (χ2) was used to compare categorical variables across groups. Kaplan-Meier analysis and Cox regression were used to assess the BCFI and OS. The impact of different prognostic factors on BCFI and OS, as well as interactions between chemotherapy benefit and those prognostic factors, were examined by Cox proportional hazards regression. We performed PSM analysis by using R program version 3.6.3. The command matched two patients with chemotherapy to one patient without chemotherapy using factors such as age, histology, tumor size, grade and Ki67, and the caliper value of PSM was 0.1.
Results
Baseline characteristics of patients in full cohort and PSM cohort
A total of 1,113 patients meeting the inclusion criteria were enrolled in this study (Figure 1): 928 received chemotherapy (74.8% choosing anthracycline- and taxan-based regimens), and 185 received no chemotherapy. Among patients treated with adjuvant chemotherapy, 209 cases were T1a-b and 719 cases were T1c. Among patients without adjuvant chemotherapy, 88 cases were T1a-b, and 97 cases were T1c. The patients’ characteristics are listed in Table 1. Compared with untreated patients, patients who received adjuvant chemotherapy displayed unfavorable features, such as young age (<40 years old, 12.6% vs. 7.6%; P=0.004), premenopausal status (43.0% vs. 31.9%, P=0.005), higher invasive ductal carcinoma (IDC) tumor proportion (89.8% vs. 80.0%, P<0.001), larger tumor size (T1cN0M0, 77.5% vs. 52.4%, P<0.001), higher tumor grade (grade III, 50.0% vs. 28.6%, P<0.001), and higher Ki67 index (Ki67 ≥14%, 86.2% vs. 61.6%, P<0.001). No significant difference was found between patients with and without adjuvant chemotherapy in terms of breast surgery type and usage of adjuvant radiotherapy.

Table 1
| Characteristics | Full cohort (N=1,113) | Propensity score matched (N=441) | |||||
|---|---|---|---|---|---|---|---|
| With chemotherapy (N=928) | Without chemotherapy (N=185) | P value | With chemotherapy (N=294) | Without chemotherapy (N=147) | P value | ||
| Age (years) | 52.0 [23–83] | 56.0 [27–85] | 0.004* | 55.0 [31–83] | 55.0 [27–85] | 0.492 | |
| <40 | 117 (12.6) | 14 (7.6) | 18 (6.1) | 13 (8.8) | |||
| 40–55 | 454 (48.9) | 77 (41.6) | 142 (48.3) | 65 (44.2) | |||
| >55 | 357 (38.5) | 94 (50.8) | 134 (45.6) | 69 (47.0) | |||
| Menstruation | 0.005* | 0.477 | |||||
| Premenopausal | 399 (43.0) | 59 (31.9) | 96 (32.7) | 53 (36.1) | |||
| Postmenopausal | 529 (57.0) | 126 (68.1) | 198 (67.3) | 94 (63.9) | |||
| Histology | <0.001* | 0.698 | |||||
| IDC | 833 (89.8) | 148 (80.0) | 254 (86.4) | 125 (85.0) | |||
| Non-IDC | 95 (10.2) | 37 (20.0) | 40 (13.6) | 22 (15.0) | |||
| Tumor size | <0.001* | 0.946 | |||||
| T1a-bN0M0 | 209 (22.5) | 88 (47.6) | 125 (42.5) | 63 (42.9) | |||
| T1cN0M0 | 719 (77.5) | 97 (52.4) | 169 (57.5) | 84 (57.1) | |||
| Grade | <0.001* | 0.800 | |||||
| I | 20 (2.2) | 4 (2.2) | 7 (2.4) | 2 (1.4) | |||
| II | 330 (35.6) | 59 (31.9) | 84 (28.6) | 55 (37.4) | |||
| III | 464 (50.0) | 53 (28.6) | 135 (45.9) | 49 (33.3) | |||
| NA | 114 (12.3) | 69 (37.3) | 68 (23.1) | 41 (27.9) | |||
| Ki67 | <0.001* | 0.882 | |||||
| <14% | 128 (13.8) | 71 (38.4) | 86 (29.3) | 42 (28.6) | |||
| ≥14% | 800 (86.2) | 114 (61.6) | 208 (70.7) | 105 (71.4) | |||
| Breast surgery type | 0.753 | 0.277 | |||||
| Lumpectomy | 413 (44.5) | 80 (43.2) | 122 (41.5) | 69 (46.9) | |||
| Mastectomy | 515 (55.5) | 105 (56.8) | 172 (58.5) | 78 (53.1) | |||
| Adjuvant radiotherapy | 0.115 | 0.485 | |||||
| Yes | 358 (38.6) | 60 (32.4) | 112 (38.1) | 51 (34.7) | |||
| No | 570 (61.4) | 125 (67.6) | 182 (61.9) | 96 (65.3) | |||
Data are shown as median [range] or n (%). *, P<0.05. IDC, invasive ductal carcinoma; NA, not available.
After matching based on the propensity score, 294 patients with chemotherapy and 147 patients without chemotherapy were identified (Table 1). The adjuvant chemotherapy group consisted of 125 T1a-b and 169 T1c patients. Among patients without adjuvant chemotherapy, 63 cases were T1a-b, and 84 cases were T1c. All baseline characteristics including age, menstruation, pathological type, tumor size, histological grade, Ki67, breast surgery type and usage of radiotherapy were comparable after PSM (P>0.05).
Prognostic factor analysis of patients in full cohort and PSM cohort
The median follow-up time of full cohort was 43.0 months. Twenty-one patients died from all causes and 65 BCFI events were recorded. In univariate analysis (Table S1), adjuvant chemotherapy was significantly associated with BCFI (P=0.026) and apt to be significant for OS (P=0.060). Results of multivariate analysis for BCFI and OS are shown in Table S2. T1c tumors were significantly associated with worse BCFI [hazard ratio (HR) =2.311; 95% confidence interval (CI): 1.167–4.578; P=0.016], while no significant association was observed for OS (P=0.123). Moreover, BCFI (HR =2.336; 95% CI: 1.217–4.484; P=0.011) and OS (HR =3.427; 95% CI: 1.186–9.899; P=0.023) decreased with the absence of adjuvant chemotherapy.
Among patients in the PSM cohort, with a median follow-up of 39.6 months, 14 patients died from all causes and 38 BCFI events were recorded. Univariate analysis showed that tumor size was the only factor influencing BCFI (P=0.037) and apt to be significant for OS (P=0.060) (Table 2). In multivariate analysis (Table 3), T1c tumors were associated with shortened BCFI (HR =2.452; 95% CI: 1.120–5.366; P=0.025), and was inclined to be related with worse OS (HR =3.479; 95% CI: 0.890–13.606; P=0.073). Regarding chemotherapy, no statistical difference was observed between the chemotherapy group and the non-chemotherapy group in terms of BCFI (P=0.187) or OS (P=0.521). Other clinical and biological features, such as age, menstruation, pathological type, tumor grade, Ki67, breast surgery type and usage of radiotherapy, were not associated with BCFI or OS.
Table 2
| Characteristics | P value | |
|---|---|---|
| BCFI | OS | |
| Age (years) (<40 vs. 40–55 vs. >55) | 0.589 | 0.293 |
| Menstruation (pre- vs. post-menopausal) | 0.993 | 0.467 |
| Tumor size (T1a-b vs. T1c) | 0.037* | 0.060 |
| Histology (IDC vs. non-IDC) | 0.280 | 0.115 |
| Grade (I vs. II vs. III) | 0.900 | 0.073 |
| Ki67 (<14% vs. ≥14%) | 0.418 | 0.766 |
| Breast surgery type (lumpectomy vs. mastectomy) | 0.457 | 0.235 |
| Adjuvant chemotherapy (yes vs. no) | 0.241 | 0.509 |
| Adjuvant radiotherapy (yes vs. no) | 0.370 | 0.110 |
*, P<0.05. BCFI, breast cancer-free interval; OS, overall survival; PSM, propensity score matched; IDC, invasive ductal carcinoma.
Table 3
| Characteristics | BCFI | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.650 | 0.771 | |||
| <40 | 1.00 (reference) | 1.00 (reference) | |||
| 40–55 | 0.661 (0.188–2.331) | 0.520 | 2,490.501 (0–1.280E+51) | 0.889 | |
| >55 | 0.484 (0.102–2.291) | 0.360 | 5,266.136 (0–2.755E+51) | 0.879 | |
| Menstruation | 0.447 | 0.628 | |||
| Pre-menopausal | 1.00 (reference) | 1.00 (reference) | |||
| Post-menopausal | 1.508 (0.523–4.346) | 0.578 (0.063–5.288) | |||
| Tumor size | 0.025* | 0.073 | |||
| T1a-bN0M0 | 1.00 (reference) | 1.00 (reference) | |||
| T1cN0M0 | 2.452 (1.120–5.366) | 3.479 (0.890–13.606) | |||
| Histology | 0.216 | 0.865 | |||
| IDC | 1.00 (reference) | 1.00 (reference) | |||
| Non-IDC | 0.413 (0.102–1.678) | 0 (0–3.375E+36) | |||
| Grade | 0.638 | 0.335 | |||
| I | 1.00 (reference) | 1.00 (reference) | |||
| II | 0.467 (0.056–3.919) | 0.483 | 11,575.021 (0–4.832E+127) | 0.949 | |
| III | 0.302 (0.032–2.836) | 0.295 | 3,093.830 (0–1.301E+127) | 0.956 | |
| Ki67 | 0.481 | 0.816 | |||
| <14% | 1.00 (reference) | 1.00 (reference) | |||
| ≥14% | 1.368 (0.572–3.271) | 1.159 (0.334–4.021) | |||
| Breast surgery type | 0.072 | 0.395 | |||
| Lumpectomy | 1.00 (reference) | 1.00 (reference) | |||
| Mastectomy | 0.640 (0.320–1.310) | 0.475 (0.086–2.640) | |||
| Adjuvant chemotherapy | 0.187 | 0.521 | |||
| Yes | 1.00 (reference) | 1.00 (reference) | |||
| No | 1.648 (0.785–3.459) | 1.479 (0.447–4.896) | |||
| Adjuvant radiotherapy | 0.080 | 0.083 | |||
| Yes | 1.00 (reference) | 1.00 (reference) | |||
| No | 1.490 (0.430–2.470) | 8.823 (0.753–103.318) | |||
*, P<0.05. BCFI, breast cancer-free interval; OS, overall survival; PSM, propensity score matched; HR, hazard ratio; CI, confidence interval; IDC, invasive ductal carcinoma.
Association between chemotherapy and survival outcomes in PSM cohort
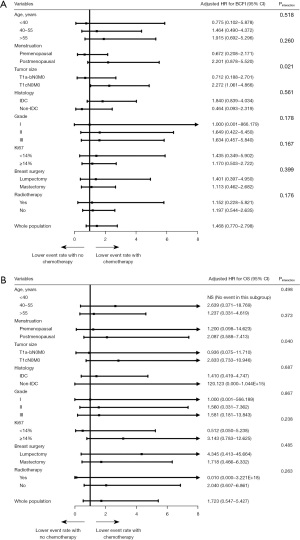
The association between chemotherapy and survival outcomes in PSM cohort is shown in Figure 2. Patients with or without chemotherapy had similar BCFI (5-year BCFI: 92.8% vs. 87.7%, P=0.241) and OS (5-year OS: 97.7% vs. 94.2%, P=0.509) (Figure 2A,2D). Regarding patients with different tumor sizes, chemotherapy did not improve BFCI (5-year BCFI: 93.6% vs. 94.6%, P=0.546) or OS (5-year OS: 97.9% vs. 98.0%, P=0.470) for T1a-b patients (Figure 2B,2E). For the T1c population, patients receiving chemotherapy had significantly better BCFI (5-year BCFI: 92.1% vs. 79.5%, P=0.035) than patients who were not receiving chemotherapy (Figure 2C). No significant OS difference was observed (5-year OS: 95.7% vs. 89.3%, P=0.159) between patients with or without chemotherapy (Figure 2F) in the T1c population. We further divided T1a-b patients into T1a and T1b groups (Figure 3). Chemotherapy was also not associated with improved BCFI both in T1a (P=0.138) and T1b population (P=0.691) (Figure 3A,3B). As for OS, no events existed in T1a population and no significant difference was observed between T1b patients with and without chemotherapy (P=0.503) (Figure 3C,3D). Further subgroup analysis indicated that tumor size was the only interacting factor determining the effect of adjuvant chemotherapy on BCFI (Pinteraction=0.021, Figure 4A) and OS (Pinteraction=0.040, Figure 4B).


Discussion
In the present study, we evaluated the survival benefit of adjuvant chemotherapy with a relatively large cohort consisting of 1,113 T1N0M0 TNBC patients from a multicenter database. After applying PSM analysis, we found that tumor size was the most important factor in determining the effectiveness of adjuvant chemotherapy in T1N0 TNBC patients, which was more relevant for T1c patients. Our findings support providing adjuvant chemotherapy for TNBC patients with T1c tumors, but the benefit of adjuvant chemotherapy for T1a-bN0 TNBC patients needs further clinical evaluation.
Traditionally, tumor stage was supposed to be significantly associated with BC patients’ prognosis and risk of recurrence (22). Previous studies did not focus on T1 small tumors intentionally or even excluded them when examining the effect of adjuvant chemotherapy on TNBCs (12-15,23,24). Recommendations from current guidelines for adjuvant chemotherapy usage in T1N0 TNBCs are also inconsistent. Adjuvant chemotherapy, which is recommended for T1b patients by the ESMO and St. Gallen guidelines, should not be used on patients with low-risk features according to the NCCN guideline. For T1a patients, the NCCN guideline recommends no chemotherapy while the ESMO and the St. Gallen guidelines recommend that chemotherapy could be considered for some patients (18-20). However, recent studies indicated that the T1N0 TNBCs might also have a metastatic potential with a relatively higher recurrence rate than other BC subtypes (25,26). Additional studies investigating the survival benefit of chemotherapy in T1N0 TNBCs have been conducted. A retrospective study with 7 T1a, 44 T1b, and 303 T1c patients showed that adjuvant chemotherapy significantly improved recurrence-free survival (RFS) significantly in T1c N0M0 TNBC patients, but not in T1a or T1b patients (27). Another single-center study including 308 patients with chemotherapy and 42 patients without chemotherapy also indicated that T1c patients could significantly benefit from adjuvant chemotherapy in RFS, but T1a or T1b patients could not (28). Similarly, de Nonneville et al. found that adjuvant chemotherapy was not associated with a significant benefit in disease-free survival (DFS) or metastasis-free survival (MFS) in T1a-b TNBCs (29). A nationwide study from Netherlands Cancer Registry also showed that adjuvant chemotherapy did not improve breast cancer-specific survival (BCSS) in T1a or T1b TNBC patients (30).
Considering that some previous studies may be underpowered given the relatively small number of T1a-b patients or patients receiving no chemotherapy, we implemented PSM to make our study cohort balanced, and our results are partially in line with the NCCN guidelines and consistent with some of the previous findings, which suggested that adjuvant chemotherapy significantly improved BCFI in T1c, but not in T1a-b TNBC patients. Regarding OS, although no significant benefit of chemotherapy was shown in either the T1a-b or T1c groups, we observed that the curves of OS in the T1c group split gradually with time going on, which indicated that T1c TNBC patients might benefit from chemotherapy with increasing observational time. Furthermore, our subgroup analysis showed an interaction between chemotherapy and tumor size for BCFI and OS, which also supported this hypothesis. In addition, our results are similar with the Surveillance, Epidemiology, and End Results (SEER) database study presented at American Society of Clinical Oncology (ASCO) annual meeting in 2023, which showed that chemotherapy improved BCSS only in T1c but not in T1a or T1b TNBC patients (31). One possible explanation of our results is that the prognosis of T1N0 TNBCs is favorable so that it is difficult to detect a survival benefit from adjuvant chemotherapy, especially for T1a-b patients. In our study, T1a-b patients without chemotherapy had excellent survival with a 5-year BCFI of 93.6% and a 5-year OS of 97.9%. Another likely reason is that the toxic side effects of chemotherapy may offset its survival benefits in small TNBCs (32).
In addition, based on the results of our study, it is particularly important to explore novel biomarkers, other than tumor size, such as stromal tumor-infiltrating lymphocytes (sTILs), programmed cell death-ligand 1 (PD-L1), BRCA mutations and androgen receptor (AR), which all play a vital role in predicting prognosis of TNBCs, to predict adjuvant chemotherapy benefit in small TNBCs. Recent studies have shown that a high presence of sTILs is associated with a better response to neoadjuvant chemotherapy as well as prognosis in T1N0 TNBCs (33-35). Moreover, TNBC patients with positive PD-L1 tend to be associated with high sTILs, and those patients with PD-L1 positivity and sTILs ≥10% are linked with a better prognosis (36,37). A multicentric retrospective study found a prolonged DFS and a trend toward prolonged disease-specific survival (DSS) among TNBC patients with BRCA1 or BRCA2 mutations (38). Similarly, AR positivity in TNBCs is significantly associated with improved DFS and a trend toward better OS (39). Therefore, these novel biomarkers are expected to be incorporated in future research and clinical practices, which may be helpful in guiding the use of chemotherapy in small TNBCs.
The strengths of our study are the detailed clinical and outcome data representing patients’ real life from SJTU-BCDB, which is a nationwide registered database in China, providing updated data from real clinical setting. Another strength of our study is that by implementing PSM, we were able to minimize the impact of confounding variables. Moreover, unlike other studies, we used BCFI rather than BCSS so that we would not omit the local, regional, and distant recurrence data, which are important in BC prognosis (30,40). However, our study also has some limitations. First, the follow-up time was relatively short (43.0 months in the full cohort and 39.6 months in the PSM cohort) for us to observe the long-term effect of adjuvant chemotherapy. Second, we were unable to differentiate the survival results from various chemotherapy regimens since the majority of patients in our cohort received anthracycline- and taxan-based regimens.
Conclusions
Our study demonstrated that tumor size was significantly associated with the survival benefit of adjuvant chemotherapy in T1N0 TNBC patients. Our data do not support the routine use of chemotherapy in patients with T1a-bN0 TNBC, and benefit of adjuvant chemotherapy for T1a-bN0 patients needs further clinical evaluation.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-189/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-189/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-189/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-189/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the independent Ethical Committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (No. 2020-0309). Informed consent was waived by the ethics committee due to its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Won KA, Spruck C. Triple-negative breast cancer therapy: Current and future perspectives Int J Oncol 2020;57:1245-61. (Review). [Crossref] [PubMed]
- Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674-90. [Crossref] [PubMed]
- Monaco ML, Idris OA, Essani K. Triple-Negative Breast Cancer: Basic Biology and Immuno-Oncolytic Viruses. Cancers (Basel) 2023;15:2393. [Crossref] [PubMed]
- Bianchini G, De Angelis C, Licata L, et al. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol 2022;19:91-113. [Crossref] [PubMed]
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012;367:1998-2005. [Crossref] [PubMed]
- Fisher B, Dignam J, Tan-Chiu E, et al. Prognosis and treatment of patients with breast tumors of one centimeter or less and negative axillary lymph nodes. J Natl Cancer Inst 2001;93:112-20. [Crossref] [PubMed]
- Gorshein E, Klein P, Boolbol SK, et al. Clinical significance of HER2-positive and triple-negative status in small (≤ 1 cm) node-negative breast cancer. Clin Breast Cancer 2014;14:309-14. [Crossref] [PubMed]
- Theriault RL, Litton JK, Mittendorf EA, et al. Age and survival estimates in patients who have node-negative T1ab breast cancer by breast cancer subtype. Clin Breast Cancer 2011;11:325-31. [Crossref] [PubMed]
- Ho AY, Gupta G, King TA, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer 2012;118:4944-52. [Crossref] [PubMed]
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Benson JR, Jatoi I, Keisch M, et al. Early breast cancer. Lancet 2009;373:1463-79. [Crossref] [PubMed]
- Lluch A, Barrios CH, Torrecillas L, et al. Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01). J Clin Oncol 2020;38:203-13. [Crossref] [PubMed]
- Mayer IA, Zhao F, Arteaga CL, et al. Randomized Phase III Postoperative Trial of Platinum-Based Chemotherapy Versus Capecitabine in Patients With Residual Triple-Negative Breast Cancer Following Neoadjuvant Chemotherapy: ECOG-ACRIN EA1131. J Clin Oncol 2021;39:2539-51. [Crossref] [PubMed]
- Gerber B, Loibl S, Eidtmann H, et al. Neoadjuvant bevacizumab and anthracycline-taxane-based chemotherapy in 678 triple-negative primary breast cancers; results from the geparquinto study (GBG 44). Ann Oncol 2013;24:2978-84. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- Fu WF, Chen QX, Wang XX, et al. The Survival Outcomes of T1aN0M0 Triple-Negative Breast Cancer With Adjuvant Chemotherapy. Front Oncol 2020;10:1753. [Crossref] [PubMed]
- Zhai Z, Zheng Y, Yao J, et al. Evaluation of Adjuvant Treatments for T1 N0 M0 Triple-Negative Breast Cancer. JAMA Netw Open 2020;3:e2021881. [Crossref] [PubMed]
- Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:691-722. [Crossref] [PubMed]
- Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 2019;30:1541-57. [Crossref] [PubMed]
- Cardoso F, Kyriakides S, Ohno S, et al. Electronic address: clinicalguidelines@esmo.org. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1194-220. Erratum in: Ann Oncol 2019;30:1674 Erratum in: Ann Oncol 2021;32:284. [Crossref] [PubMed]
- American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2018.
- Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005;5:591-602. [Crossref] [PubMed]
- de Jong VMT, Wang Y, Ter Hoeve ND, et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy. J Clin Oncol 2022;40:2361-74. [Crossref] [PubMed]
- Hahnen E, Lederer B, Hauke J, et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol 2017;3:1378-85. [Crossref] [PubMed]
- Al-Mahmood S, Sapiezynski J, Garbuzenko OB, et al. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res 2018;8:1483-507. [Crossref] [PubMed]
- Gamucci T, Vaccaro A, Ciancola F, et al. Recurrence risk in small, node-negative, early breast cancer: a multicenter retrospective analysis. J Cancer Res Clin Oncol 2013;139:853-60. [Crossref] [PubMed]
- Ren YX, Hao S, Jin X, et al. Effects of adjuvant chemotherapy in T1N0M0 triple-negative breast cancer. Breast 2019;43:97-104. [Crossref] [PubMed]
- An X, Lei X, Huang R, et al. Adjuvant chemotherapy for small, lymph node-negative, triple-negative breast cancer: A single-center study and a meta-analysis of the published literature. Cancer 2020;126:3837-46. [Crossref] [PubMed]
- de Nonneville A, Gonçalves A, Zemmour C, et al. Adjuvant chemotherapy in pT1ab node-negative triple-negative breast carcinomas: Results of a national multi-institutional retrospective study. Eur J Cancer 2017;84:34-43. [Crossref] [PubMed]
- Steenbruggen TG, van Werkhoven E, van Ramshorst MS, et al. Adjuvant chemotherapy in small node-negative triple-negative breast cancer. Eur J Cancer 2020;135:66-74. [Crossref] [PubMed]
- Tarantino P, Leone J, Vallejo CT, et al. Prognosis and trends in chemotherapy use for patients with stage Ia triple-negative breast cancer (TNBC): a population-based study. J Clin Oncol 2023;41:abstr 510.
- Oladeru OT, Singh AK, Ma SJ. Association of adjuvant chemotherapy with overall survival among women with small, node-negative, triple-negative breast cancer. JAMA Netw Open 2020;3:e2016247. [Crossref] [PubMed]
- Loi S, Drubay D, Adams S, et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol 2019;37:559-69. [Crossref] [PubMed]
- Park JH, Jonas SF, Bataillon G, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol 2019;30:1941-9. [Crossref] [PubMed]
- Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40-50. [Crossref] [PubMed]
- Loi S, Michiels S, Adams S, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol 2021;32:1236-44. [Crossref] [PubMed]
- Carter JM, Polley MC, Leon-Ferre RA, et al. Characteristics and Spatially Defined Immune (micro)landscapes of Early-stage PD-L1-positive Triple-negative Breast Cancer. Clin Cancer Res 2021;27:5628-37. [Crossref] [PubMed]
- De Talhouet S, Peron J, Vuilleumier A, et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep 2020;10:7073. [Crossref] [PubMed]
- Thike AA, Yong-Zheng Chong L, Cheok PY, et al. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol 2014;27:352-60. [Crossref] [PubMed]
- Zheng YZ, Liu Y, Deng ZH, et al. Determining prognostic factors and optimal surgical intervention for early-onset triple-negative breast cancer. Front Oncol 2022;12:910765. [Crossref] [PubMed]


