Laparoscopic sleeve gastrectomy for the treatment of diabetes mellitus type 2 patients—single center early experience
Introduction
The main answer to the worldwide obesity epidemic is the increase in the number of performed bariatric procedures. Type 2 diabetes and glucose metabolism abnormalities are one the most important effects of morbid obesity which lead to severe and chronic reduction in quality of health. High effectiveness of bariatric therapy for weight reduction and treatment of comorbidities has been proven in numerous studies (1). But it is yet still unclear which bariatric procedure should be chosen for diabetic patients in order to achieve the best results in diabetes remission (2).
In recent years, laparoscopic sleeve gastrectomy (LSG) has become one of the most commonly used primary bariatric procedure for morbid obesity (3,4). Numerous authors strive to prove that effect of LSG on type 2 diabetes treatment is as good as laparoscopic Roux-en-Y gastric bypass (LRYGB), which was known as a “gold standard” for diabetic patients. Potential mechanisms of diabetes remission and improvement in glucose homeostasis after LSG are the main topic of recent studies, yet its results are still unclear (5). While LRYGB has well documented positive clinical influence on type 2 diabetes, the role of LSG in diabetes treatment is debatable. Many studies show great biochemical results. Although this should induce diabetes remission, the clinical long-term results are not so optimistic.
Aim of study
The main aim of this study is to present our early experience in LSG as a method of bariatric treatment in patients with type 2 diabetes or abnormalities in glucose homeostasis. The secondary aim of our study is identification of potential preoperative predictors of diabetes remission and glucose homeostasis improvement.
Methods
Prospectively collected data of patients operated for morbid obesity at the 2nd Department of Surgery, Jagiellonian University Medical College were analyzed. Guidelines of the Metabolic and Bariatric Surgery Section of the Polish Surgical Society were used as criteria for surgical treatment, i.e., body mass index (BMI) ≥35 kg/m2 with obesity comorbidities, or BMI ≥40 kg/m2, with or without comorbidities. All patients included in the study underwent LSG. The study was designed to assess the influence of LSG on type 2 diabetes and glucose homeostasis. The primary endpoint was the diabetes type 2 remission, which was described as glycosylated hemoglobin—HbA1c <6% (42 mmol/mol) without the use of diabetes medications. Secondary endpoint was the change of glucose metabolism parameters after LSG. Patients were assessed preoperatively and allocated to two groups: group 1—with any preoperative abnormalities in glucose homeostasis (prediabetes, diabetes) and group 2—with non-elevated fasting glucose level. To identify potential preoperative factors which can predict results of metabolic outcome, typical glucose homeostasis parameters were analyzed: fasting glucose level, insulin, proinsulin, C-peptide, HOMA-IR, HOMA-B and HbA1c%. During follow-up (6 months after surgery) all glucose homeostasis parameters were analyzed again.
Statistical analysis was performed using STATISTICA 10.0 PL. Data are presented as median values with inter-quartile range. Chi-square exact Fisher test, Pearson and Yates tests were used to compare of qualitative data. The t-test and Mann-Whitney, Wilcoxon, Cochrane-Cox tests analyzed quantitative differences between groups. Data were found statistically significant with P value of 0.05.
Ethical statement: the study was approved by the ethics review committee of the Jagiellonian University (approval number KBET/156/B/2011) and written informed consent was obtained from all patients.
Material
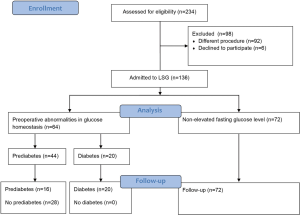
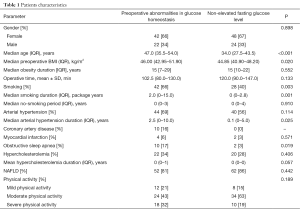
Two hundred and thirty-six patients underwent laparoscopic bariatric procedures at 2nd Department of General Surgery, Jagiellonian University Medical College between 2014 and 2016. One hundred and thirty-six patients after LSG were enrolled in the study (90 females, 46 males; mean age 40.5±9.9 years). Preoperative abnormalities in glucose homeostasis were confirmed in 64 (47%) patients [42 females, 22 males; mean age 47 (35.5–54) years]. Twenty (15%) patients in this group had diabetes and 44 (32%) had prediabetes defined as abnormal level of glucose homeostasis parameters. Seventy-two (53%) patients had normal fasting glucose level [48 females, 24 males; mean age 34 (27.5–43.5) years]. Median preoperative BMI in group with preoperative abnormalities in glucose homeostasis and without was respectively 46 (42.95–51.9) and 44.85 (40.9–48.2) kg/m2. Typical comorbidities were more common in the group of patients with preoperative abnormalities in glucose homeostasis. Table 1 presents groups characteristics. Patients’ flow through the study is illustrated in Figure 1.
Full table
Results
We observed significant reduction of BMI after surgery in the group with abnormalities in glucose homeostasis [46 (42.95–51.9) to 33 (29.4–38.9) kg/m2]. Comparable results we found in the group with non-elevated fasting glucose level [44.85 (40.9–48.2) to 33.3 (31.4–37.2) kg/m2]. Mean percent of EBMIL for all groups after 6 months from surgery was 59.90% (46.75–69.28%).
Unfortunately there were no full remissions after surgery in patients with preoperative diabetes. Every patients with preoperative insulin treatment remained on insulin, however the dose of insulin decreased. Of 60 (44%) patients who were preoperatively taking oral diabetic medications, only 36 (26%) need them during follow up. The number of patients with poor glycemic control decreased from 14 (70%) to 8 (40%) (Table 2).

Full table
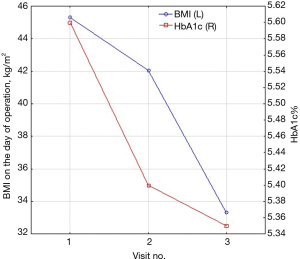
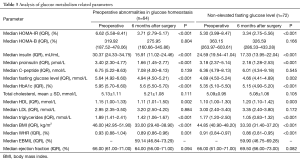
We found significant improvement in biochemical markers of glucose homeostasis. Number of patients with prediabetes significantly decreased from 44 (32.35%) to 16 (11.76%), P<0.001. Insulin resistance (HOMA-IR) in both groups (with and without abnormalities in glucose homeostasis) decreased significantly after surgery from 6.62% (5.58–8.41%) and 5.30% (3.99–8.47%). Median insulin level dropped from baseline 30.37 (24.33–34.75) to 15.91 (11.02–24.46) mU/mL in first group and from 24.59 (19.54–41.04) to 17.33 (13.95–22.04) mU/mL in the second. Proinsulin level decreased from 3.40 (2.30–4.77), 3.18 (2.37–5.14) to 1.66 (1.45–2.77), 2.18 (1.28–2.53) pmol/L respectively. There were no significant changes in postoperative levels of C-peptide. Medium level of HbA1c% before surgery in the group with preoperative abnormalities in glucose homeostasis was 5.95% (5.7–6.6%) and 5.35% (5.1–5.5%) in the group with non-elevated fasting glucose level. We observed significant reduction of HbA1c% after surgery in both groups to 5.6% (5.5–5.7%) and 5.15% (4.9–5.2%) (Table 3). The level of postoperative HbA1c% was related to BMI loss after surgery (Figure 2).
Full table
A univariate logistic regression analysis showed that preoperative age (OR, 9.92; 95% CI, 0.87–0.97; P=0.003), fasting glucose level (OR, 0.5; 95% CI, 0.29–0.87; P=0.013), HbA1c% (OR, 0.19; 95% CI, 0.06–0.61; P=0.004), total cholesterol level (OR, 2.2; 95% CI, 1.24–3.91; P=0.007) and LDL (OR, 2.57; 95% CI, 1.28–5.16; P=0.006) were related with postoperative diabetes remission and improvement of glucose metabolism (Table 4).
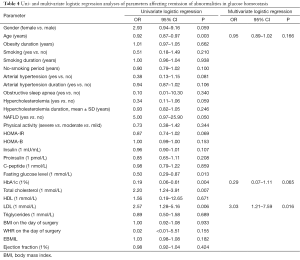
Full table
Discussion
LRYGB and LSG are currently the most common bariatric surgeries in Poland (6). The role of bariatric surgery in treatment of morbid obesity is well established in our country. The effect of bariatric surgeries on weight reduction is important as well as its impact on comorbidities, especially type 2 diabetes. In the age of bariatric surgery, type 2 diabetes can be viewed as a curable disease. Bariatric surgery has been confirmed to be beneficial in remission of abnormalities in glucose homeostasis (7). Type 2 diabetes is an indication for bariatric surgery if patient’s BMI exceeds 35 kg/m2. Patients with BMI >30 and <35 kg/m2 may be considered for metabolic surgery on an individual basis (8,9). Unfortunately, no surgical guidelines for bariatric treatment or any statements of international diabetes organization define what kind of surgery would best for diabetic patients with morbid obesity (9-11).
Due to very good long-term effects on weight reduction and remarkable resolution of comorbidities and improvements in glucose homeostasis, LRYGB formerly regarded as absorptive procedure became a standard procedure. However it is a difficult procedure, with numerous early and late complications and high risk of malnutrition in the future. For this reason many authors try to use different techniques to treat diabetic patients. LSG is technically easier, and the newest data suggest occurrence of some important metabolic changes after operation. Nowadays LSG is no more defined as an only restrictive procedure (2).
In the literature we can find confusing data about weight loss results after LRYGB and LSG. In some articles authors present higher percent of EWL after LRYGB. Our observation revealed that the weight reduction after both procedures is similar (12). In our study percent of EBMIL after LSG, measured 6 months after surgery, was 59.90% (46.75–69.28%) and it was comparable to others authors (13,14).
In opposition to LRYGB, mechanisms of diabetes remission after LSG are not well-defined. The GLP-1 play the key-role in changes of glucose metabolism and it is responsible for improvement of glucose homeostasis after LRYGB. After LSG the level of GLP-1 rises as well, thus it has been suggested to contribute to potential improvements in diabetes remission (15). In our previous studies we noticed the same relations between gut hormones after LSG (16).
Numerous authors present satisfactory biochemical results which should be related with diabetes remission. Unfortunately long-term clinical observations are not so encouraging. Similarly, in our study after 6 months from the surgery we noticed significant remission of biochemical abnormalities of glucose homeostasis, however we did not cure diabetes. Twenty patients with type 2 diabetes before surgery still need medical treatment for glycemic control. Jammu and Sharma in their group described remission of type 2 diabetes in 13 of 23 patients (17). Sixty-seven percent of diabetes remission after LSG was presented in the study of Milone, who compared it with results after mini gastric bypass (18). All patients who preoperatively needed insulin still need it, but at a lower dose. All patients reduced oral diabetic medications. We noticed improvement in biochemical glucose homeostasis, which was described as significant changes of HOMA-IR, level of insulin, C-peptide and HbA1c% after 6 months. Despite that there were no cases of complete remission of diabetes. Similar results and very rare diabetes remission was presented by Aminian (19).
The most important issue in assessment of metabolic effects of bariatric surgery is the criteria for diabetes remission. This creates space for potential biases. It can explain the differences found in the literature.
Nevertheless even if patient did not meet the clinical criteria of complete remission of type 2 diabetes, the most important metabolic profit after surgery is improvement in glycemic control. It can be noticed in the level of HbA1c% after surgery, which reduced significantly. Our observation refers to both, group with abnormalities in glucose homeostasis and the group with non-elevated fasting glucose level. Vigneshwaran et al. present similar observations. In their study level of HbA1c% decreased from (8.7±1.6)% to (6.7±1.5)% (20). Interestingly the level of HbA1c% was correlated to percent of EBMIL in contrast to results presented in the Milone study (18).
The preoperative information about potential predictors of postoperative glycemic abnormalities remission can lead to improvement of long-term effects. In “ABCD score” we can find some potential factors (age, BMI, C-peptide, diabetes duration) which can be useful to predict diabetes remission after bariatric surgery (21). In our study we tried to confirm this relationship and identify some new factors, which can be related with better or worse metabolic answer after LSG. We noticed that only age, fasting glucose level, HbA1c%, total cholesterol level and LDL level were statistically important for remission of abnormalities in glucose homeostasis. Age and HbA1c% seem to be the most important factors. Similar to our observation, Milone et al. considered HbA1c% as a negative predictor of diabetes remission (18). In the study of Hamza, chance for diabetes remission was reduced by 20% with each additional 12 years of age (22). Older age and worse glycemic control, defined as a higher level of HbA1c%, are the negative predictors for diabetes remission. In patients with such condition LRYGB should be recommended.
Conclusions
LSG leads to significant improvement in biochemical glucose homeostasis and can be considered as a method of treatment in morbidly obese patients with glucose metabolism abnormalities. LSG as a method of treatment for patients with clinical type 2 diabetes still needs some further observation. In elderly patients with poorly compensated type 2 diabetes LRYGB should be recommended.
Long term observations from double-blinded randomized control trials will be helpful to make the final decision which procedure should be considered in candidates for bariatric treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board of the Jagiellonian University (No. KBET/156/B/2011) and written informed consent was obtained from all patients.
References
- Sundbom M. Laparoscopic revolution in bariatric surgery. World J Gastroenterol 2014;20:15135-43. [Crossref] [PubMed]
- Li J, Lai D, Wu D. Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy to Treat Morbid Obesity-Related Comorbidities: a Systematic Review and Meta-analysis. Obes Surg 2016;26:429-42. [Crossref] [PubMed]
- Nguyen NT, Nguyen B, Gebhart A, et al. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. J Am Coll Surg 2013;216:252-7. [Crossref] [PubMed]
- Matłok M, Pędziwiatr M, Major P, et al. One hundred seventy-nine consecutive bariatric operations after introduction of protocol inspired by the principles of enhanced recovery after surgery (ERAS®) in bariatric surgery. Med Sci Monit 2015;21:791-7. [Crossref] [PubMed]
- Murphy R, Evennett NJ, Clarke MG, et al. Sleeve gastrectomy versus Roux-en-Y gastric bypass for type 2 diabetes and morbid obesity: double-blind randomised clinical trial protocol. BMJ Open 2016;6:e011416. [Crossref] [PubMed]
- Janik MR, Stanowski E, Paśnik K. Present status of bariatric surgery in Poland. Videosurgery Miniinv 2016;11:22-5. [Crossref]
- Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 2014;24:42-55. [Crossref] [PubMed]
- Yumuk V, Tsigos C, Fried M, et al. European Guidelines for Obesity Management in Adults. Obes Facts 2015;8:402-24. [Crossref] [PubMed]
- Rubino F, Nathan DM, Eckel RH, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016;39:861-77. [Crossref] [PubMed]
- Garvey WT, Mechanick J, Brett EM, et al. American association of clinical endocrinologists and american college of endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity - executive summary. Endocr Pract 2016;22:842-84. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002-13. [Crossref] [PubMed]
- Major P, Matłok M, Pędziwiatr M, et al. Quality of Life After Bariatric Surgery. Obes Surg 2015;25:1703-10. [Crossref] [PubMed]
- Arman GA, Himpens J, Dhaenens J, et al. Long-term (11+years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Schneider J, Peterli R, Gass M, et al. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: a prospective randomized trial. Surg Obes Relat Dis 2016;12:563-70. [Crossref] [PubMed]
- Garibay D, McGavigan AK, Lee SA, et al. β-Cell Glucagon-Like Peptide-1 Receptor Contributes to Improved Glucose Tolerance After Vertical Sleeve Gastrectomy. Endocrinology 2016;157:3405-9. [Crossref] [PubMed]
- Major P, Matłok M, Pędziwiatr M, et al. Changes in levels of selected incretins and appetite-controlling hormones following surgical treatment for morbid obesity. Wideochir Inne Tech Maloinwazyjne 2015;10:458-65. [Crossref] [PubMed]
- Jammu GS, Sharma R. A. 7-Year Clinical Audit of 1107 Cases Comparing Sleeve Gastrectomy, Roux-En-Y Gastric Bypass, and Mini-Gastric Bypass, to Determine an Effective and Safe Bariatric and Metabolic Procedure. Obes Surg 2016;26:926-32. [Crossref] [PubMed]
- Milone M, Di Minno MN, Leongito M, et al. Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol 2013;19:6590-7. [Crossref] [PubMed]
- Aminian A, Brethauer SA, Andalib A, et al. Can Sleeve Gastrectomy "Cure" Diabetes? Long-term Metabolic Effects of Sleeve Gastrectomy in Patients With Type 2 Diabetes. Ann Surg 2016;264:674-81. [Crossref] [PubMed]
- Vigneshwaran B, Wahal A, Aggarwal S, et al. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30-35.0 kg/m2: a Prospective Study. Obes Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Lee MH, Lee WJ, Chong K, et al. Predictors of long-term diabetes remission after metabolic surgery. J Gastrointest Surg 2015;19:1015-21. [Crossref] [PubMed]
- Hamza N, Abbas MH, Darwish A, et al. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surg Obes Relat Dis 2011;7:691-6. [Crossref] [PubMed]