
Literature review and guide for optimal position in implant-based breast reconstruction
Introduction
The goal of immediate reconstruction after mastectomy is to maximize quality of life, provide an optimal aesthetic outcome, mitigate complications, and restore functionality to support the patient’s activity level. Identification of ideal candidates for prepectoral versus retropectoral implant-based breast reconstruction relies on careful preoperative risk assessment and intraoperative flap evaluation. Preoperative risk assessment requires consideration of all factors and comorbidities that may be associated with greater risk for development of complications or poor aesthetic reconstructive outcomes, which include body mass index (BMI), breast volume, ptosis, skin quality and other contributing factors including intraoperative skin flap quality (1-3). The plastic surgeon’s primary goal is to provide superior aesthetic outcomes to ensure psychosocial wellbeing, improved quality of life, and overall high patient satisfaction. Once determined that implant-based breast reconstruction (IBR) is oncologically appropriate for the patient, there are few consolidated resources to guide the surgeon’s decision-making process when evaluating the preferred plane (prepectoral versus retropectoral) for implant placement. This article provides a guide for the reconstructive surgeon to make a comprehensive decision on how to choose between the prepectoral and retropectoral planes for implant-based breast reconstruction after mastectomy. It is based on literature review, personal clinical experience, and evaluation of each step of the pre- and postoperative situation. We present this article in accordance with the Narrative Review reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-78/rc).
Methods
An analysis of PubMed studies was used for the literature review from a search performed between May 2022 and February 2023. The key words and phrases used to conduct the search included implant-based breast reconstruction, prepectoral, retropectoral, macromastia, ptosis and/or complications. Using the bibliography of each article, additional relevant articles not found in the PubMed search were identified for use in the literature review. The studies included were restricted to those reported in the English language, with no restrictions based on year of publication, though newer studies were assigned higher priority. Randomized control trials were favored, followed by cohort studies (prospective and retrospective, respectively) and case reports were excluded from the PubMed search. The included articles were then independently assessed by sample size, patient characteristics, reconstructive technique including plane utilized for implant placement, average size of implant, and complication rates prior to inclusion in this literature review. The literature review process is further outlined in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | May 2022 to February 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | Implant-based breast reconstruction, prepectoral, retropectoral, macromastia, ptosis and/or complications |
| Timeframe | No restrictions based on year of publication, though newer studies were assigned higher priority |
| Inclusion and exclusion criteria | Inclusion: English language |
| Exclusion: case reports, studies deemed insufficient based on author discretion | |
| Selection process | Literature review and selection by authors CAK, MKM, EAT, CAS |
Discussion
Patient characteristics and preferences may influence surgeon decision-making during preoperative assessment of reconstructive options (Figure 1). We discuss herein factors identified in clinical practice and in literature review that guide clinical decision-making for plane of reconstruction and surgical technique, as well as our developed guides for preoperative plane selection and intraoperative flap assessment.
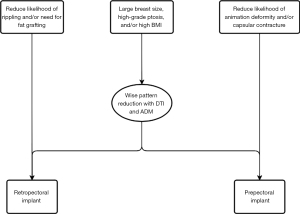
BMI, breast volume, and ptosis
When selecting the appropriate plane for implant-based reconstruction, the surgeon must take into account the patient’s breast size, degree of ptosis, and BMI to tailor the surgical approach to achieve optimal patient-centered outcomes. Across all forms of reconstruction, Hanwright et al. reported higher overall morbidity in patients with high BMI among 12,986 patients (4). Other studies demonstrated concordant findings that high rates of overall complications are associated with high BMI in patients undergoing implant-based reconstruction compared to patients with lower BMI (5-8). No studies have formally delineated optimal plane based on BMI, but rather used flap thickness as a predictor of reconstructive success. In patients with macromastia and ptosis, the challenge of a large skin envelope invites the need for a skin reduction procedure preceding reconstruction. The most common method to achieve adequate skin reduction in patients with large or ptotic breasts is the Wise-pattern technique, with comparable complication rates reported for implants placed in the retropectoral and prepectoral planes (5,9,10). However, Patel et al. reported a minor increase in infection rates when using ADM with tissue expanders (TEs) compared to ADM and implants (11). They concluded that a deepithelialized dermal flap was sufficient to support implants without ADM for those undergoing Wise-pattern reduction-reconstruction. Similarly, in a study examining outcomes in three groups (prepectoral, retropectoral with ADM, or retropectoral with no ADM) of 294 reconstructions, Bettinger et al. demonstrated that BMI >40 kg/m2 was associated with increased complication rates associated with tissue expander placement, such as prosthetic loss, seroma, hematoma, infection, skin necrosis, and nipple necrosis (12). Thus, careful patient selection to mitigate these types of complications is required to achieve optimal results, and surgery without the use of expanders may be recommended in this specific population.
History of radiation therapy
Prior radiation has been associated with increased fibrosis, vascular changes, and changes in elasticity of the skin and surrounding subcutaneous tissues that may compromise future reconstruction efforts (13-15). Olinger et al. detailed an experience of 1,594 patients, of whom 84 underwent prior breast conserving therapy (BCT) with radiotherapy as well as 329 who underwent postmastectomy radiation therapy (PMRT) (16). Results showed no difference in rates of reconstructive failure among those with prior BCT compared to PMRT, but 4.28 greater odds of failure compared to those with no radiation exposure (P<0.001). Parsa et al. demonstrated no difference in outcomes in 27 previously irradiated breasts compared to contralateral non-irradiated breasts following delayed reconstruction after mastectomy based on radiation exposure alone (17). However, while breasts with moderate skin changes and no induration experienced equivalent outcomes to nonirradiated breasts, breasts with severe skin changes or presence of induration predicted greater likelihood of capsular contracture. Additionally, Kearney et al. revealed that patients with prior radiotherapy were more likely to convert to autologous reconstruction compared to patients with no history of radiation, though they exhibited similar complication profiles across groups with respect to necrosis, prosthetic failure, cellulitis, hematoma, and seroma (18). These studies highlight the importance of shared decision-making between both the multidisciplinary team and the patient, with an emphasis on the potential risk of reconstructive failure in the context of radiation history.
PMRT & capsular contracture
In patients requiring PMRT, prepectoral implant placement with ADM prior to treatment therapy has become one attempt to mitigate capsular contracture. Two large meta-analyses both demonstrated significantly reduced odds of capsular contracture with prepectoral reconstruction compared to retropectoral approaches [Li et al.: odds ratio (OR): 0.45; 95% confidence interval (CI): 0.27–0.73 and Abbate et al.: OR: 0.48; 95% CI: 0.28–0.81] (19,20). Sinnott et al. demonstrated among 426 breasts an increase (and higher Baker grades >3) in capsular contracture among patients undergoing retropectoral reconstruction compared to prepectoral (52.2% vs. 16.1%; P=0.0018), thought to be due to increased direct irradiation of the implant and surrounding capsule with less ADM coverage with their retropectoral technique (21). Several studies have alluded to the benefit of ADM when protecting against capsular contracture (22). In patients undergoing prepectoral reconstruction, aggregated data of 27 studies showed a decrease in capsular contracture rates from 12.4% to 2.3% with the inclusion of ADM (23). Other studies have demonstrated successful avoidance of capsular contracture in patients with retropectoral reconstruction with greater ADM coverage. Salzberg et al. demonstrated low overall capsular contracture rates (1.9%) in a population of retropectorally reconstructed breasts with ADM coverage, but 7.1 times greater odds of developing capsular contracture in postoperatively irradiated breasts compared to no radiation (24). The study also demonstrated increased odds of capsular contracture with implant size <400 mL compared to ≥400 mL (OR: 10.30, P=0.0008). Sbitany et al. demonstrated no difference in capsular contracture among a small cohort of 24 breasts undergoing PMRT when stratifying by plane of reconstruction, both of which incorporated ADM coverage (25). Further, Nava et al. revealed that PMRT on tissue expanders was associated with significantly higher rates of totally failed reconstruction with 40 percent of unsuccessful reconstructions compared with 6.4 percent on permanent implants (26). Ultimately, careful patient selection depending on postoperative radiation therapy needs and the amount of ADM that will be incorporated is crucial for prevention of these complications.
Complication prevention and treatment
While complication mitigation is ideally always the surgeon’s goal and improved surgical practices have reduced complication rates to historically low levels, it is essential to address and correct complications as they arise. Complications associated with placement of the implants in one plane may be corrected with a change to the other plane. For example, animation deformity, a potential consequence of retropectoral reconstruction, and rippling, most commonly associated with prepectoral implant placement, may be treated with transfer of the prosthesis to the opposite plane.
Animation deformity
Several studies report up to 77.8% of women who have undergone retropectoral reconstruction experience some degree of animation deformity, a defect in which a prosthesis is laterally displaced upon flexion of the pectoralis major muscle (27-30). While tolerable in some patients, many patients are distressed by symmetry concerns or pain, particularly when exercising or lifting weights, and previous studies have demonstrated patient interest in alternative initial procedures that would have avoided animation deformity altogether (27,28). Current literature suggests that animation deformity was associated with negative impacts on breast aesthetics and quality of life. In a study by Becker et al., 80% of patients reported that the deformity is bothersome and 48% reported that the deformity interrupted activities of daily life (29). Several studies demonstrated that revision surgery to the prepectoral plane was a viable treatment for animation deformity. Gabriel et al. showed complete resolution of animation deformity in 100% of their 57 patients who underwent revisionary plane change to the prepectoral position with ADM coverage, with a relatively low complication rate of 3.9% (31). King et al. demonstrated complete resolution of animation deformity in 21 retropectoral reconstructions with plane change revision (32). In a prospective study evaluating animation deformity in 37 patients based on plane of reconstruction, Dyrberg et al. found mean incidence of animation deformity in the retropectoral group to be much higher compared to prepectoral as evaluated by two independent observers on the Nipple, Surrounding Skin, Entire Breast (NSE) grading scale (4 vs. 0.2; P<0.001) (33). Two systematic reviews aggregated findings from existing literature on animation deformity. The first reported an overall incidence of 58% among 996 patients across four studies for some degree of animation deformity, most commonly associated with two retropectoral reconstruction or augmentation techniques, which were Regnault and dual-plane (34). Among 13 studies evaluating 1,894 patients, the second review noted a 73.9% prevalence of animation deformity in retropectoral implant-based reconstructions and augmentations compared to 10.5% in the prepectoral plane (35). Additionally, the group reported on grading systems for animation deformity and identified nine different grading systems, of which Kim et al. and Dyrberg et al. were noted to be highest in quality and highly reproducible for clinical use (33,35,36). However, they acknowledged that perhaps one of the most important factors to assess animation deformity is the patient’s perception of severity, a concept that was evaluated by Becker et al. demonstrating a moderate positive correlation between clinical grade and patient-perceived severity, with Pearson’s correlation coefficient of 0.47 and P value of 0.0145 (29). These studies collectively highlight the need for mitigation of animation deformity when possible as well as careful evaluation of patient perception of severity to maximize overall quality of life.
Contour abnormalities, rippling, and autologous fat grafting
Autologous fat grafting has become a cornerstone in treatment of contour abnormalities in implant-based breast reconstruction. The procedure has been effective in addressing the step-off with prepectoral implants to create a more natural-appearing breast slope (37). Rippling, a type of contour abnormality from direct implant ripple visualization that typically arises more prominently with prepectoral reconstruction, can be concealed by autologous fat grafting, whereby the patient’s own fat harvested from a donor site is injected into the site of concern. Fat grafting procedures have been demonstrated to be effective in treatment of rippling, with good cosmetic outcomes, high overall patient satisfaction on the BREAST-Q survey, and reduced pain (37,38). Rippling may also arise in patients who have undergone retropectoral reconstruction, though the prevalence is generally lower compared to prepectoral (32). While plane change to the retropectoral position is an option to mitigate the perception of rippling, autologous fat grafting is an effective approach that requires no dissection. However, counseling on the average number of fat grafting procedures needed to achieve the desired cosmetic result is essential to set realistic expectations for the patient during their treatment. A prior study has demonstrated an average of 2.2 fat grafting procedures regardless of plane of reconstruction for those who choose to undergo further treatment (38). Additionally, fat grafting is not ideal in patients who are thin or have had previous graft failures (37). The procedure is associated with higher rates of calcifications, fat necrosis, oil cyst formation, and fat resorption, and thus is not without risk for future complications.
Implant displacement/bottoming out
While implant displacement can be a distressing symptom for patients, the frequency of this complication has diminished with the advent of ADM (39). ADM provides increased support and stability of the implant, particularly at the inferior pole to prevent bottoming out, and ensures the prosthesis remains in the preferred position (39). In cases where ADM cannot be used or is insufficient, implant displacement can be corrected with capsulorrhaphy with variable success and longevity, whereby the capsule is sutured closed for additional prosthesis security (40).
Flap perfusion
Impaired perfusion to mastectomy skin flaps with implant-based breast reconstruction may result in problems with wound healing and mastectomy skin flap necrosis. When considering how much tension the mastectomy flap can withstand, preoperative evaluation must consider risk assessment of all factors that might affect perfusion. Established patient risks factors include obesity, diabetes, hypertension, history of stroke, tobacco use, and radiation therapy (41). Flap morbidity was identified to be associated with elevated BMI and increased breast volumes (42). Though not an absolute contraindication, prior radiation therapy is associated with higher rates of complication including loss of prosthesis (18.75%) and infection (21.6%) (43). Clinical assessment of the irradiated skin should be performed, and autologous reconstruction is recommended for patients who have developed severe skin changes or induration following radiation (44). Patients who develop mild skin changes following radiation are more likely to have a successful outcome (45). Factors such as incision type, skin flap thickness and mastectomy weight have also been shown to impact mastectomy skin flap viability. The reported ideal candidate for favorable outcomes in single-stage implant-based breast reconstruction is a nonsmoking patient with low BMI, no history of diabetes, grade 1 or 2 ptosis, and mastectomy weight less than 500 g with the desire for equal or smaller breast size (41,44,46-48). Additionally, flap thickness is at the discretion of the breast surgical oncologist who performs the mastectomy dissection to provide the ideal oncologic result. Oncologic safety of mastectomy relies on breast surgeon expertise of flap thickness to ensure adequate removal of glandular tissue (49,50). Andersson et al. revealed that flap thickness >5 mm greatly increases the amount of residual breast tissue (51). However, Frey et al. demonstrated a significant increase in ischemic complications with flaps <8 mm (52). These collective data demonstrate the difficulty in reconciling maximal glandular tissue resection and maintenance of adequate blood supply to the flap. Working collaboratively, the breast surgeon and the plastic surgeon must communicate about realistic options for maximizing oncologic and aesthetic outcomes.
Surgery-related risk factors for impaired mastectomy skin flap perfusion including duration of surgery, incision type, skin flap thickness, and mastectomy weights have been investigated in the literature. When preparing the breast skin envelope intraoperatively, the oncologic surgeon must balance obtaining negative margins and achieving adequate thickness to maintain skin flap viability (53). Careful assessment of flap thickness, skin discoloration, capillary refill, and dermal bleeding is important to evaluate viability of the skin flap. Longer surgical times and use of the Wise-pattern breast reduction techniques have been shown to be associated with higher rates of mastectomy flap necrosis (53). In mastectomies performed with Wise-pattern incisions, there is a risk of delayed wound healing and mastectomy flap necrosis in up to 30% of cases secondary to the combination of thin, long skin flaps that are easily devascularized and the presence of a T-junction (54). Flap thickness less than 8 mm has also been found to be an independent predictor of ischemic complications (52). Mastectomy weight has also been described as a risk factor for skin flap ischemia with a reported 1.6-fold increased risk of major skin complications for every 100 g increase in mastectomy weight (54). Implant weight greater than 468 g was significantly associated with skin flap ischemic complications and should be avoided if possible (55).
Several objective tools have been developed as adjuncts to support the clinical judgment of the reconstructive surgeon in assessing tissue perfusion intraoperatively. The intravenous sodium fluorescein test involves intravenous injection of fluorescein dye followed by intraoperative evaluation of skin fluorescence under Wood’s lamp illumination. This is a test of vascularity and its application is limited by subjective errors and changes in blood supply and has become more historical in nature. Laser-assisted indocyanine green dye angiography (LA-ICGA) can identify poorly perfused areas in the skin flap and become the standard intraoperative tool for blood flow assessment. A SPY Elite value of ≤7 accurately predicted the development of mastectomy flap necrosis with 88% sensitivity and 83% specificity (52). In comparison to fluorescein dye, indocyanine green dye is superior for prediction of mastectomy flap necrosis (52). Harless et al. reported an 86% decrease in the rate of mastectomy skin flap necrosis after implementation of LA-ICGA (56). Optical diffusion imaging spectroscopy is another tool that obtains a noninvasive, real-time measurement of tissue hemoglobin oxygen saturation (StO2) using the ratio of oxyhemoglobin to deoxyhemoglobin (57). Selective application of these tools can demonstrate skin flap areas with suboptimal perfusion for removal to reduce wound complications and optimize reconstructive outcomes.
Limitations
This review includes a careful selection of articles that the authors feel is representative of the existing literature. Not all articles on the topic were included in this study, which may result in differing narratives, though the overall principles and techniques that inform the conclusions should stay relatively unchanged. Ideally, the preferred study design for research studies included in any review would be a randomized controlled trial, however, selection of the plane during reconstruction is a highly specialized and personal decision between patient and provider, and thus randomization in this setting may not be appropriate. As a result, this review included a large number of cohort studies based on the published studies available on this subject. While shorter term outcomes are well defined, future investigations should examine longer term patient-reported outcomes with regard to satisfaction, pain, and quality of life for both planes.
Recommendations
Prepectoral versus retropectoral reconstruction decision-making algorithm
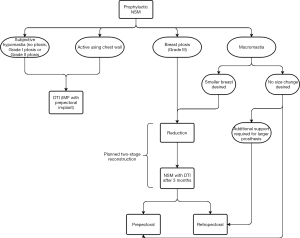
Clinical decision-making algorithms for direct-to-implant (DTI) reconstruction with acellular dermal matrix (ADM) for patients undergoing prophylactic or therapeutic nipple-sparing mastectomy (NSM) were developed based on patient characteristics, surgical techniques and outcomes. Certain factors are taken under consideration to evaluate whether a patient is best suited for one of three surgical approaches, which includes DTI reconstruction with ADM, two-staged reconstruction with TEs and ADM, or delayed reconstruction, as well as consideration of which plane is optimal. The preoperative selection algorithm is separated into two decision-making processes based on indication for NSM, whether prophylactic or therapeutic, followed by a third decision tree to guide intraoperative assessment.
Preoperative reconstruction assessment for NSM
An algorithm to guide reconstruction technique was developed for patients undergoing prophylactic NSM (Figure 2). If the patient has subjective hypomastia, Grade I or II ptosis, or the patient has an active chest wall (i.e., pursues athletic activities with chest involvement), they may be an ideal candidate for DTI implant-based breast reconstruction with inframammary fold (IMF) incision and prepectoral placement. Patients with an active chest wall are recommended to have implants placed prepectorally to avoid adverse outcomes with animation deformity. For patients with macromastia with no change in breast size desired, retropectoral reconstruction may recommended to provide additional support for a larger prosthesis. If the patient has macromastia with desire for smaller breast size, or a patient has Grade III ptosis, they are recommended to undergo a planned two-stage reconstruction with Wise-pattern bilateral reduction mammoplasty followed by DTI reconstruction at least 3–6 months later. Plane of reconstruction is then typically decided based on desired implant volume: if the implants are smaller, the patient may be a good candidate for prepectoral reconstruction, whereas if the implants are larger and require additional support, the patient may be recommended to undergo retropectoral reconstruction, in order to provide more overall support and prevent malposition and descent. The patient requiring or desiring implants greater than 400 cc should be evaluated carefully for proper support, with 400 cc serving as a guideline and not a formal cutoff based on current literature reporting implant size greater than 400 to be an independent predictor of complications during reconstruction (58).
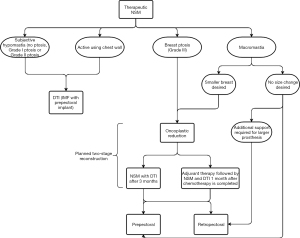
An analogous algorithm for preoperative assessment of reconstruction options in patients undergoing therapeutic NSM was developed (Figure 3). Patients with subjective hypomastia, grade I or II ptosis, or an active chest wall are still recommended to undergo DTI reconstruction with IMF incision in the prepectoral plane. For patients with macromastia and no desire for breast size change, they are more likely recommended to undergo retropectoral reconstruction for reinforced support of a larger implant. Patients with macromastia who desire a smaller breast size, as well as patients with grade III ptosis, should consider undergoing a planned two-stage reconstruction, beginning with oncoplastic reduction, followed by NSM with DTI reconstruction after 3–6 months or adjuvant chemotherapy followed by NSM and DTI reconstruction one month following chemotherapy completion. This group may undergo prepectoral or retropectoral reconstruction based on implant size desired with the recommendation for patients desiring smaller implants to pursue prepectoral reconstruction. Those desiring larger implants may undergo prepectoral reconstruction but should also consider retropectoral placement for additional prosthesis support.
Intraoperative decision-making for both prophylactic and therapeutic NSM
An intraoperative decision algorithm was developed for all patients undergoing implant-based reconstruction regardless of reconstructive plane (Figure 4). All patients, regardless of preoperative reconstructive plan, are recommended to undergo intraoperative assessment, preferably with indocyanine green angiography (ICG) or other perfusion assessment tools. In both prophylactic and therapeutic NSMs, if intraoperative assessment demonstrates marginal flap perfusion (<30% perfusion), the surgeon can opt to delay reconstruction or perform a two-staged reconstruction with TEs and ADM. Conversely, if intraoperative assessment reveals healthy perfusion of the flap, DTI reconstruction with ADM is recommended in all groups per the preoperative plan.

Conclusions
Careful preoperative and intraoperative assessment of reconstruction options for patients undergoing implant-based breast reconstruction is necessary to mitigate complications and produce superior aesthetic outcomes. In our experience, higher-risk groups for poor outcomes include those with an active chest wall, high BMI, ptosis, history of radiotherapy, and those who will undergo adjuvant radiotherapy, necessitating surgical planning tailored to risk factors to optimize outcomes and quality of life. Prepectoral reconstruction is most suitable for patients with small breasts or macromastia with desire for smaller breasts, low-grade ptosis, smaller implant size, those undergoing PMRT, and for those who aim to mitigate animation deformity and capsular contracture. Retropectoral reconstruction is recommended for patients with high-grade ptosis, or larger breasts with no desire for size change, in patients who aim to reduce likelihood of rippling and need for subsequent fat grafting procedures to address contour abnormalities, and potentially in patients desiring larger implants who must be carefully evaluated for proper support. Close collaboration with an experienced breast surgeon with a good understanding of flap thickness is crucial to produce an optimal and successful aesthetic result. Decision algorithms may be used to determine ideal surgical techniques based on patient factors.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-78/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-78/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-78/coif). CAS serves as an unpaid editorial board member of Gland Surgery from September 2022 to August 2024. EAT discloses a faculty position for Medtronic educational courses. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palve JS, Luukkaala TH, Kääriäinen MT. Predictive risk factors of complications in different breast reconstruction methods. Breast Cancer Res Treat 2020;182:345-54. [Crossref] [PubMed]
- Thorarinsson A, Fröjd V, Kölby L, et al. Patient determinants as independent risk factors for postoperative complications of breast reconstruction. Gland Surg 2017;6:355-67. [Crossref] [PubMed]
- Kobraei EM, Nimtz J, Wong L, et al. Risk factors for adverse outcome following skin-sparing mastectomy and immediate prosthetic reconstruction. Plast Reconstr Surg 2012;129:234e-41e. [Crossref] [PubMed]
- Hanwright PJ, Davila AA, Hirsch EM, et al. The differential effect of BMI on prosthetic versus autogenous breast reconstruction: a multivariate analysis of 12,986 patients. Breast 2013;22:938-45. [Crossref] [PubMed]
- Friedman HI, Talebagha S, Gilstrap J, et al. Wise Pattern Direct Implant Breast Reconstruction: A Review and Improved Outcomes Using Dermal Matrix. Plast Reconstr Surg Glob Open 2019;7:e2439. [Crossref] [PubMed]
- Roubaud MS, Carey JN, Vartanian E, et al. Breast reconstruction in the high-risk population: current review of the literature and practice guidelines. Gland Surg 2021;10:479-86. [Crossref] [PubMed]
- Rochlin DH, Nguyen DH. Deepithelialized Skin Reduction Preserves Skin and Nipple Perfusion in Immediate Reconstruction of Large and Ptotic Breasts. Ann Plast Surg 2018;81:22-7. [Crossref] [PubMed]
- Cagli B, Morelli Coppola M, Augelli F, et al. Postmastectomy Radiation Therapy in the Setting of Two-Stage Retropectoral Implant-Based Breast Reconstruction: Should It be Delivered Before or After Implant Exchange? A Retrospective Analysis on 183 Patients. Aesthetic Plast Surg 2022;46:2643-54. [Crossref] [PubMed]
- Komorowska-Timek E, Merrifield B, Turfe Z, et al. Subcutaneous Prosthetic Breast Reconstructions following Skin Reduction Mastectomy. Plast Reconstr Surg Glob Open 2019;7:e2078. [Crossref] [PubMed]
- Newman MK. Reconstruction of the Ptotic Breast Using Wise Pattern Skin Deepithelialization. Plast Reconstr Surg Glob Open 2016;4:e1077. [Crossref] [PubMed]
- Patel AA, Kayaleh H, Sala LA, et al. Comparing Outcomes of Wise-Pattern, Two-Stage Breast Reduction-Reconstruction with and without Acellular Dermal Matrix. Plast Reconstr Surg 2021;148:511-21. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Cordeiro PG, Albornoz CR, McCormick B, et al. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg 2014;134:588-95. [Crossref] [PubMed]
- Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol 2014;21:118-24. [Crossref] [PubMed]
- Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol 2017;18:e742-53. [Crossref] [PubMed]
- Olinger TA, Berlin NL, Qi J, et al. Outcomes of Immediate Implant-Based Mastectomy Reconstruction in Women with Previous Breast Radiotherapy. Plast Reconstr Surg 2020;145:1029e-36e. [Crossref] [PubMed]
- Parsa AA, Jackowe DJ, Johnson EW, et al. Selection criteria for expander/implant breast reconstruction following radiation therapy. Hawaii Med J 2009;68:66-8. [PubMed]
- Kearney AM, Brown MS, Soltanian HT. Timing of radiation and outcomes in implant-based breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:1719-26. [Crossref] [PubMed]
- Li Y, Xu G, Yu N, et al. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Meta-analysis. Ann Plast Surg 2020;85:437-47. [Crossref] [PubMed]
- Abbate O, Rosado N, Sobti N, et al. Meta-analysis of prepectoral implant-based breast reconstruction: guide to patient selection and current outcomes. Breast Cancer Res Treat 2020;182:543-54. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]
- Mangialardi ML, Salgarello M, Cacciatore P, et al. Complication Rate of Prepectoral Implant-based Breast Reconstruction Using Human Acellular Dermal Matrices. Plast Reconstr Surg Glob Open 2020;8:e3235. [Crossref] [PubMed]
- Wagner RD, Braun TL, Zhu H, et al. A systematic review of complications in prepectoral breast reconstruction. J Plast Reconstr Aesthet Surg 2019;72:1051-9. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg 2011;128:353-9. [Crossref] [PubMed]
- Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44-8. [Crossref] [PubMed]
- Nigro LC, Blanchet NP. Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1407. [Crossref] [PubMed]
- Becker H, Fregosi N. The Impact of Animation Deformity on Quality of Life in Post-Mastectomy Reconstruction Patients. Aesthet Surg J 2017;37:531-6. [Crossref] [PubMed]
- Alnaif N, Safran T, Viezel-Mathieu A, et al. Treatment of breast animation deformity: A systematic review. J Plast Reconstr Aesthet Surg 2019;72:781-8. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthet Surg J 2018;38:519-26. [Crossref] [PubMed]
- King CA, Bartholomew AJ, Sosin M, et al. A Critical Appraisal of Late Complications of Prepectoral versus Subpectoral Breast Reconstruction Following Nipple-Sparing Mastectomy. Ann Surg Oncol 2021;28:9150-8. [Crossref] [PubMed]
- Dyrberg DL, Gunnarsson GL, Bille C, et al. A simple clinical assessment of breast animation deformity following direct-to-implant breast reconstruction. Arch Plast Surg 2019;46:535-43. [Crossref] [PubMed]
- Dyrberg DL, Bille C, Gunnarsson GL, et al. Breast animation deformity. Arch Plast Surg 2019;46:7-15. [Crossref] [PubMed]
- Dalaei F, Dyrberg DL, Bille C, et al. An update on breast animation deformity grading systems—a systematic review. Ann Breast Surg 2022;6:26. [Crossref]
- Kim JYS, Qiu CS, Chiu WK, et al. A Quantitative Analysis of Animation Deformity in Prosthetic Breast Reconstruction. Plast Reconstr Surg 2019;144:291-301. [Crossref] [PubMed]
- Darrach H, Kraenzlin F, Khavanin N, et al. The role of fat grafting in prepectoral breast reconstruction. Gland Surg 2019;8:61-6. [Crossref] [PubMed]
- Cogliandro A, Barone M, Tenna S, et al. The Role of Lipofilling After Breast Reconstruction: Evaluation of Outcomes and Patient Satisfaction with BREAST-Q. Aesthetic Plast Surg 2017;41:1325-31. [Crossref] [PubMed]
- Macadam SA, Lennox PA. Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Can J Plast Surg 2012;20:75-89. [Crossref] [PubMed]
- Spear SL. Little JW3rd. Breast capsulorrhaphy. Plast Reconstr Surg 1988;81:274-9. [Crossref] [PubMed]
- Wink JD, Fischer JP, Nelson JA, et al. Direct-to-implant breast reconstruction: an analysis of 1612 cases from the ACS-NSQIP surgical outcomes database. J Plast Surg Hand Surg 2014;48:375-81. [Crossref] [PubMed]
- Luze H, Nischwitz S, Wurzer P, et al. Assessment of mastectomy skin flaps for immediate reconstruction with implants via thermal imaging—A suitable, personalized approach? J Pers Med 2022;12:740. [Crossref] [PubMed]
- Sbitany H, Wang F, Peled AW, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg 2014;134:396-404. [Crossref] [PubMed]
- Rodriguez-Feliz J, Codner MA. Embrace the Change. Plast Reconstr Surg 2015;136:221-31. [Crossref] [PubMed]
- Salzberg CA. Direct-to-implant breast reconstruction. Clin Plast Surg 2012;39:119-26. [Crossref] [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. The nipple-sparing mastectomy: early results of a feasibility study of a new application of perioperative radiotherapy (ELIOT) in the treatment of breast cancer when mastectomy is indicated. Tumori 2003;89:288-91. [Crossref] [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol 2012;23:2053-8. [Crossref] [PubMed]
- Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg 2013;132:1043-54. [Crossref] [PubMed]
- Storm-Dickerson T, Sigalove NM. The breast surgeons’ approach to mastectomy and prepectoral breast reconstruction. Gland Surg 2019;8:27-35. [Crossref] [PubMed]
- Robertson SA, Jeevaratnam JA, Agrawal A, et al. Mastectomy skin flap necrosis: challenges and solutions. Breast Cancer (Dove Med Press) 2017;9:141-52. [Crossref] [PubMed]
- Andersson MN, Sund M, Svensson J, et al. Prophylactic mastectomy - Correlation between skin flap thickness and residual glandular tissue evaluated postoperatively by imaging. J Plast Reconstr Aesthet Surg 2022;75:1813-9. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation using Magnetic Resonance Imaging. Plast Reconstr Surg Glob Open 2017;5:e1439. [Crossref] [PubMed]
- Abedi N, Ho AL, Knox A, et al. Predictors of Mastectomy Flap Necrosis in Patients Undergoing Immediate Breast Reconstruction: A Review of 718 Patients. Ann Plast Surg 2016;76:629-34. [Crossref] [PubMed]
- Di Candia M, Lie KH, Forouhi P, et al. Experience with the Wise mammaplasty skin resection pattern in skin-sparing mastectomy and immediate breast reconstruction for large breast volumes. Int J Surg 2011;9:41-5. [Crossref] [PubMed]
- Santanelli F, Longo B, Sorotos M, et al. Flap survival of skin-sparing mastectomy type IV: a retrospective cohort study of 75 consecutive cases. Ann Surg Oncol 2013;20:981-9. [Crossref] [PubMed]
- Harless CA, Jacobson SR. Tailoring through Technology: A Retrospective Review of a Single Surgeon's Experience with Implant-Based Breast Reconstruction before and after Implementation of Laser-Assisted Indocyanine Green Angiography. Breast J 2016;22:274-81. [Crossref] [PubMed]
- Rao R, Saint-Cyr M, Ma AM, et al. Prediction of post-operative necrosis after mastectomy: a pilot study utilizing optical diffusion imaging spectroscopy. World J Surg Oncol 2009;7:91. [Crossref] [PubMed]
- Choi M, Frey JD, Alperovich M, et al. "Breast in a Day": Examining Single-Stage Immediate, Permanent Implant Reconstruction in Nipple-Sparing Mastectomy. Plast Reconstr Surg 2016;138:184e-91e. [Crossref] [PubMed]


