
Sensory recovery and the role of innervated flaps in autologous breast reconstruction—a narrative review
Introduction
Background
Reconstructive surgery is an integrated part of breast cancer care, and continues to be refined. Native breast skin and nipple are increasingly spared, while maintaining oncological safety. Moreover, microsurgical advances have enabled a wide range of autologous breast reconstruction options, yielding permanent and satisfying results (1,2). Both contribute positively to aesthetic outcomes after breast cancer surgery. Nevertheless, functional outcomes fall behind as sensation recovers poorly in a reconstructed breast.
The significance of breast sensation to patients is evident from previous research and media attention. The loss of sensation is unpleasant and unanticipated, as illustrated by the New York Times (2017) article “After Mastectomies, an unexpected blow: Numb new breasts” (3,4). It also affects functional integrity of the breast, which consequently increases susceptibility to injury (5). This advocates restoring sensation in the reconstructed breast.
Sensory nerve coaptation has been proposed as a possible solution to improve postoperative sensation. The first innervated autologous breast reconstruction was described in 1992 by Slezak et al., using the fourth lateral intercostal nerve and a mixed abdominal nerve in TRAM flaps (6). Later, this technique was modified to spare the motor branches. Allen and Treece first applied this technique to Deep Inferior Epigastric artery Perforator (DIEP) flaps (7). More recently, Spiegel et al. proposed a novel technique using the anterior branch of the third intercostal nerve as recipient nerve (8). However, undervaluation of its importance and a lack of scientific support has prohibited wide implementation of innervated breast reconstruction.
Rationale and knowledge gap
In the last decade, a renewed interest in sensory recovery after breast reconstruction resulted in vastly increasing public and scientific interest. Currently, sensory nerve coaptation is increasingly considered a meaningful addition to modern era autologous breast reconstruction, both in immediate as well as delayed autologous breast reconstruction (9). Multiple systematic reviews unanimously demonstrate that sensory nerve coaptation improves postoperative breast sensation, and secondarily improves patient-reported quality of life (10-14). It has, however, still not been adopted as standard care.
Objective
In this narrative review, the current knowledge and understanding of innervated autologous breast reconstruction is explored, highlighting its significance and clinical implications. The purpose of this paper is to contribute to a widespread clinical implementation to make innervated flaps the new gold standard of autologous breast reconstruction. We present this article in accordance with the Narrative Review reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-40/rc).
Methods
A thorough PubMed search was conducted consisting of a multiplicity of synonyms and relevant MeSH-terms surrounding the keywords “sensation”, “innervated” and “autologous breast reconstruction”. Additionally, a manual search of referenced articles was conducted. As it comprises most relevant medical literature, no other databases were searched besides PubMed and references of included articles. Only original articles written in English were considered. The titles and abstracts were screened for relevance by the authors. All remaining articles were compiled as source material for this narrative review (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | October 31, 2022; updated January 30, 2023 |
| Databases and other sources searched | PubMed; manual search of references |
| Search terms used | “sensation” AND “innervated” AND “autologous breast reconstruction” (with accompanying synonyms and MeSH terms) |
| Timeframe | Unrestricted |
| Inclusion and exclusion criteria | Included: original and review articles (published or accepted for publication) |
| Excluded: other article types, language other than English | |
| Selection process | Two reviewers (JMB and JAFVR) independently reviewed and selected the articles. Relevant information was extracted |
Results
In this comprehensive overview of sensory recovery in breast reconstruction we consecutively address three defined situations. We first describe fundamental and clinical knowledge about sensation in a non-operated breast and relevant donor sites, as knowledge about normal anatomy and physiology is imperative to interpret and improve sensation. After breast surgery, sensation is altered due to disruption of the nerves in the operative field. Therefore, the second part covers the alteration of sensation after breast surgery. This also comprises sensory recovery after breast reconstruction. In the third and final part, techniques to restore sensation and outcomes in the reconstructed breast are described and critically discussed.
Sensation in the normal breast and donor sites
Anatomy—breast
The anterior thoracic wall is innervated by several different nerves: the intercostal nerves, the pectoral nerves, the long thoracic nerve, and the supraclavicular nerves (15). The cutaneous innervation of the breast is provided by the intercostal nerves, with a small contribution of the supraclavicular nerves at the superior part of the breast (16). The intercostal nerves derive from anterior branches of the 1st through 11th thoracic spinal nerves. They extend alongside the intercostal arteries and veins at the inferior costal margins and consist of two cutaneous systems: anterior and lateral. These anterior and lateral cutaneous branches innervate the thoracic wall (Figure 1) (17).
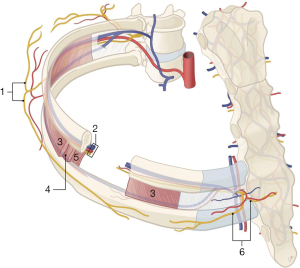
Anatomical and in vivo studies indicate that the breast skin is innervated bilaterally by the anterior and lateral cutaneous branches of the second through sixth intercostal nerves (Figure 2). Its largest surface area is covered by the fourth intercostal nerve. The nipple areolar complex (NAC) similarly receives innervation from anterior and lateral nerve branches, but this involves a multiplicity of nerves rather than merely one individual nerve. Most prevalent are the third through fifth intercostal nerves (18,19).

Anatomy—donor site
The abdominal skin is innervated by the anterior cutaneous branches of the 7th through 12th thoracic spinal nerves, which course through the rectus muscle (20). The anatomy of abdominal sensory nerves is variable. Most sensory nerves lie laterally in the suprafascial plane, whereas most mixed nerves lie subfascially and are centered around the midline (21).
Non-abdominal donor sites include the back, buttock and thigh. The back and flanks are innervated by cutaneous branches of thoracic and lumbar spinal nerves (22,23). The gluteal region by dorsal branches of lumbar segmental nerves and the posterior cutaneous femoral nerve (24). The thigh is innervated by the anterior femoral cutaneous nerve and cutaneous branches of the obturator nerve anteriorly and medially, by the posterior femoral cutaneous nerve posteriorly, and by the lateral femoral cutaneous nerve laterally (25,26).
Quantitative outcomes—breast
The nervous system is divided into the central and peripheral nervous system. Sensory receptors transmit external stimuli via the peripheral nervous system to the central nervous system. A variety of receptors are present, each detecting distinct sensory signals. The receptors associate with different types of nerve fibers (Aβ, Aδ, C). Sensory assessment tools can reflect the function of different nerve fibers of the peripheral nervous system (27).
Tactile sensation has been assessed to establish normative values and confounding factors for breast sensation (28-30). Higher age, larger breasts, previous pregnancies and breastfeeding, and higher body mass index (BMI) negatively influence breast sensation. Contrary to common assumptions, the NAC appears less sensitive than surrounding breast skin (31,32).
In the central nervous system, sensation can be evaluated using functional magnetic resonance imaging (fMRI). Hereby, cortical representations of external stimuli are measured (33,34). This perspective is a novelty in evaluating breast sensation. The breast is localized between the groin and the first digit on the primary somatosensory cortex; predominantly in the contralateral hemisphere (35,36). Recent work showed that the representation of the breast follows a somatotopic organization similar to other body parts, with distinct representation of the medial and lateral side, and the nipple. Cortical magnification of the nipple was observed, comparable to other sensitive body parts with a high receptor density (37). These studies provide insight into breast sensation in the central nervous system.
Quantitative outcomes—donor site
The current arsenal of autologous reconstruction techniques comprises various donor sites. An important consideration related to sensory outcomes is that each donor site has its own intrinsic physiological characteristics including sensory properties. Different donor sites possess different baseline characteristics regarding sensory thresholds and receptor density. A comparison between various donor sites revealed that tactile thresholds of the abdomen resemble those of the native breast most accurately (38). Contrarily, the thigh and buttock have significantly inferior sensation, with the lateral thigh area being the least sensitive. These findings may clinically affect sensory outcomes after innervated breast reconstruction.
Patient-reported outcomes—breast
Besides quantitative measurements, the subjective appraisal of breast sensation takes other valuable facets of sensation into account. Patient-reported outcomes (PROs) and qualitative research are useful to explore those additional facets of sensation.
A phenomenological study by Cornelissen et al. in healthy women identified several interrelated themes related to the qualitative appraisal of breast sensation (4). The ‘absent’ breast was most often reported, meaning that women are not actively aware of (sensation in) their breasts on a daily basis. However, all women indicated they would miss breast sensation if it were absent. It furthermore enhances the feeling of safety, as it indicates intact functionality and the ability to notice pain and warning signals.
Besides this qualitative appraisal of sensation, PROs are increasingly important. Patient-centered care and shared decision making are highly valued clinical standards in this era. The BREAST-Q score is widely implemented clinically and scientifically to address patient-reported outcomes in breast cancer patients. A novel Sensation module was developed and published in 2021 by Tsangaris et al., enabling clinicians and researchers to better monitor and understand the patient’s perspective on breast sensation (39).
Patient-reported outcomes—donor site
Currently, no qualitative outcome measures regarding the (preoperative) donor site exist. The preoperative BREAST-Q domains do not include the donor site nor its sensation.
Clinical implications
Knowledge about normal breast sensation serves two clinically relevant purposes. First, in patients with immediate breast reconstruction it reflects the preoperative situation. It therefore determines what clinicians can expect during preoperative examination, and it can guide clinicians when educating patients about breast sensation. Second, when attempting to restore sensation to as ‘normal’ as possible, sensation in healthy and unoperated breasts serves as reference and aids the interpretation of surgical outcomes.
In addition, awareness about patients’ subjective appraisal of sensation enables clinicians to specifically address those topics that are meaningful to patients during outpatient consultation. Eventually, this will likely improve patient satisfaction by creating realistic expectations. Routine use of PRO instruments such as the BREAST-Q, starting preoperatively, is encouraged to better understand and monitor patient satisfaction and quality of life.
Sensation in the operated breast
Anatomy
While part of the nerves innervating the chest wall can be preserved during mastectomy, depending on the type of mastectomy as well, the majority of sensory nerves that innervate the breast skin are transected. This is especially the case for the lateral cutaneous branches of the intercostal nerves that provide sensory innervation of the breast. These nerves excite from under the fourth to sixth rib, enter the glandular breast tissue in the deep plane, and travel under the thoracodorsal vessels (40). The anterior cutaneous branches of the intercostal nerves, on the other hand, present in the subcutaneous plane and can occasionally be spared during skin-sparing and nipple-sparing mastectomy (41).
To spare the lateral intercostal nerves for coaptation, Peled et al. introduced a nerve-preserving mastectomy technique, followed by immediate implant-based breast reconstruction (42). With this technique, the lateral cutaneous branches are dissected into the breast parenchyma during nipple-sparing mastectomy until they ramify. The nerve is then sharply transected proximally, preserving maximal length of the subareolar part of the nerve. Following this, standard nipple-sparing mastectomy is performed, ensuring a sharp dissection without electrocautery under the NAC to minimize damage to the neurovascular tissue. This technique may be utilized in other forms of mastectomy, but has not been demonstrated in delayed reconstruction. The preserved nerves can be coapted during breast reconstruction, to restore sensation.
Quantitative outcomes
Sensation is severely impaired after mastectomy and breast reconstruction. After a mastectomy, tactile thresholds are significantly lower compared to preoperative sensation. After implant-based breast reconstruction these values further deteriorate, leading to a loss of protective sensation in a large surface area of the breast (43,44). Sensation to touch and temperature are both significantly diminished, which increases the susceptibility to thermal and mechanic injury (5,45,46). This is the case for immediate implant-based breast reconstruction, but likely more so in delayed implant-based breast reconstruction. This stretches the skin, reducing the number of sensation receptors per square centimeter.
Although breast sensation after autologous reconstruction is less poor than after implant-based reconstruction, it remains far from normal. The extent to which spontaneous return of sensation occurs is highly variable, inconsistent and unpredictable; and it remains considerably impaired compared to a healthy breast (47).
Patient-reported outcomes
The loss of sensation is often unanticipated by patients, and negatively influences their appreciation of the outcomes after breast reconstruction. The New York Times [2017] article “After Mastectomies, an unexpected blow: Numb new breasts” illustrated this from the patient’s perspective and created public awareness for the issue (3). A phenomenological study by de Boer et al. also concluded that postoperative sensation (or lack thereof) is the most unexpected experience after breast reconstruction. Patients often did not consider absent sensation a possible outcome, and could therefore not conceptualize this prior to their reconstruction. Therefore, this lack of sensation was a disappointing outcome (48). Unmet expectations are the fundamental source of this dissatisfaction. In psychosocial research it is well-established that expectations determine satisfaction with outcome (49,50). Pusic et al. provided a thorough overview of how this applies to many different medical fields (51). Importantly, they found that unmet expectations after breast reconstruction indeed negatively affect patient satisfaction and health-related QoL.
Clinical implications
Knowing how different surgical modalities affect postoperative breast sensation is essential for clinicians to provide patients with adequate information. Clinicians are obliged to inform patients before obtaining consent. While it is standard practice to provide information about risks and benefits, as well as esthetic outcomes, patients are still often not informed about postoperative breast sensation. Sensory outcomes and measurements provide patients with objective results on what to expect regarding postoperative loss of breast sensation. Rather than just informing patients about numbness or generally impaired sensation, patients can be provided with more detailed information, such as expected loss of protective sensation in parts of the breast.
Moreover, a close collaboration with the breast cancer surgeons is essential. Not only in preoperative care, planning, and counseling; but also during the surgery. The breast surgeon can make an effort to preserve nerve branches and ensure gentle tissue handling to optimize future sensory recovery.
As stated before, satisfaction with surgical outcomes depends considerably on preoperative expectations and whether these are met. It is therefore important as a clinician to know and educate about postoperative sensation, and to monitor patient satisfaction. Improving patient education and implementing PRO tools in clinical practice is recommended to diminish this source of patient dissatisfaction.
Restoring sensation
Anatomy—breast
Sensory nerve coaptation is a promising technique to improve postoperative sensation and thereby improve functional outcomes after autologous breast reconstruction. As recipient, several intercostal nerves are suitable (52,53). The technique proposed by Spiegel et al. in 2013, using the anterior branch of the third intercostal nerve is described below (8).
During autologous breast reconstruction, the mammary vessels are usually dissected in the third intercostal space. The anterior branch of the intercostal nerve can be easily identified. It is located at the junction of the inferior part of the third rib and the sternum. The nerve is dissected to achieve as much length as possible, and then transected. Subsequently, the donor and recipient nerves are coapted. For direct nerve coaptation we recommend 9-0 nylon epineural sutures and fibrin sealant.
Grafts or conduits may be used to aid coaptation. If the dissected nerves are of insufficient length, or in case of a significant size mismatch, direct coaptation may not be preferable. As tensionless repair is an important denominator of a successful nerve coaptation, using a graft or conduit could be beneficial. The graft or conduit serves as a scaffold that enhances and steers neuronal regeneration (54,55).
Acellular nerve allografts are an effective means to bridge the gap between the donor and recipient nerves in DIEP flap breast reconstruction, and yields satisfactory results regarding return of sensation (56). If allografts are not available, another nerve or even a vein can be used as autograft instead (57,58). Nerve conduits can be used for smaller gaps or combined with grafts, and also seem to positively affect sensory recovery (59). Supposed advantages are protection of the anastomosis, prevention of scar formation, and creation of a favorable microenvironment for axonal regeneration (8).
However, evidence for the proposed added value of nerve grafts and conduits over a direct nerve coaptation is still minimal. In addition, allografts and conduits are costly and not available in all countries. Therefore, we want to emphasize that in our experience, nerves of sufficient length can be harvested in the majority of flaps that enable direct coaptation without the necessity to use a graft or conduit.
Anatomy—donor sites
During flap dissection, the donor nerves are identified and dissected alike the perforators. For abdominal flaps, sensory nerve branches of the 10th to 12th thoracic intercostal nerve are commonly identified in proximity of the perforators. After identification, the nerves are dissected and transected at the junction where the common nerve splits into a motor and sensory branch, at fascia level. Attention is paid to preserve the motor branches. The technique for innervated DIEP flap breast reconstruction is visualized in Figure 3.
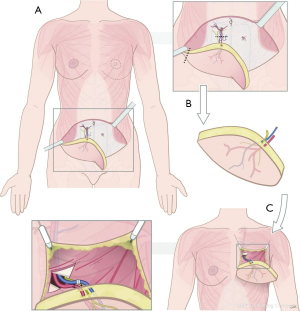
Sensory nerves of alternative donor sites can be dissected similarly. Although not yet thoroughly investigated for each, sensory nerves suitable to harvest for nerve coaptation are present in a variety of different flaps. Nerves from which those sensory nerve branches derive are summarized in Table 2 (22,24,26,60-69). The technique for innervated lateral thigh perforator (LTP) flap breast reconstruction is visualized in Figure 4.
Table 2
| Flap | Sensory nerve options | Clinical pearls |
|---|---|---|
| Deep inferior epigastric artery perforator | 10th–12th icn | Merely the sensory branches should be transected, after the mixed nerve splits into a distinct sensory and motor branch. The sensory branches pierce the anterior rectus sheath along the medial and lateral perforators through and continue into the subcutaneous tissue |
| Stacked hemi-abdominal extended perforator | 10th–12th icn and sometimes cutaneous iliohypogastric nerve | See DIEP flap innervation. If a dual innervation is not possible, innervating the superficial part of the flap is recommended |
| Latissimus dorsi | lateral branch of dorsal division of 7th thoracic nerve (sometimes 6th or 8th) | Nerves enter the flap from an upper medial oblique direction and are usually located caudally in the flap |
| Lumbar artery perforator | Superior cluneal nerves or 12th lateral icn | Cluneal nerves perforate the thoracolumbar fascia and run along the perforators |
| Superior gluteal artery perforator | Dorsal branches of lumbar segmental nerves | Multiple large caliber nerves are usually present. They are located laterally at the superior edge of the flap, cranial to the perforators |
| Septocutaneous gluteal artery perforator | 12th icn, lateral branch | The nerves pass between the internal oblique and transversus abdominis muscles, and continue toward the lateral margin of the rectus sheath from posterosuperior to anteroinferior |
| Inferior gluteal artery perforator | Posterior femoral cutaneous nerve | The nerves accompany the perforator. They are encountered in the subfascial plane along the inferior incision in the gluteal crease, and proximally in the subfascial plane during dissection of the vascular pedicle |
| Diagonal/transverse upper gracilis | Anterior femoral cutaneous nerve or obturator nerve | Sensory nerves enter the flap medially, distinct from the vascular pedicle |
| Profunda artery perforator | Posterior femoral cutaneous nerve | Nerves usually pierce the adductor magnus muscle a few centimeters lateral to the perforator |
| Lateral thigh perforator | Lateral femoral cutaneous nerve | The nerve is located at the anterior border of the flap, cranially to the septocutaneous perforator, and easy to identify |
icn, intercostal nerve; DIEP, deep inferior epigastric perforator.

Quantitative outcomes—DIEP and TRAM flaps
Innervated TRAM flap breast reconstructions consistently surpass non-innervated counterparts in overall sensory recovery (70-73). This early evidence is mainly deduced from relatively small and retrospective cohorts. Nonetheless, superior sensory recovery of innervated TRAM flaps was also confirmed in a randomized prospective study by Temple et al. (74). Tactile and thermal sensation significantly improve in innervated compared to non-innervated TRAM flap breast reconstruction. Puonti et al. also compared different surgical techniques, and concluded that innervated flaps have improved sensory recovery irrespective of the selected nerve and anastomosis technique (75). Dual nerve coaptation, in which a medial and a lateral sensory nerve of the breast are coapted, potentially further enhances sensory outcomes compared with a single nerve coaptation (53,76).
In DIEP flap breast reconstruction, results are similar. In 1999, Blondeel et al. compared sensation in innervated and non-innervated DIEP flaps (77). Tactile and thermal sensation was significantly improved in the innervated compared to non-innervated DIEP flaps. Assessment of sensory evoked potentials and patient-reported outcomes via questionnaires were also superior in the innervated flaps.
In the past decade a steep increase in the interest in innervated breast reconstruction led to the publication of methodologically improved studies. In a comparative cohort study, Beugels et al. demonstrated better sensory recovery in innervated versus non-innervated DIEP flaps (78). Also, in a study population with bilateral breast reconstruction with unilateral nerve coaptation, favorable sensory outcomes of innervated flaps were evident (79). All quadrants of the breast, all quadrants of the areola, and the nipple itself gain better postoperative tactile sensation in innervated breast reconstruction. Regardless of reconstruction timing and differences in preoperative sensory measurements, sensation of innervated flaps consistently recovers better. Beugels et al. found that nerve coaptation was significantly associated with superior sensory recovery in all areas of immediate breast reconstructions, while nerve coaptation in delayed DIEP flap breast reconstructions was only significantly associated with improved sensory recovery of the flap skin, but not of the native skin (80).
Furthermore, repeated measurements over time indicate that sensation returns not only to a larger extent, but also earlier in innervated flaps. Besides sensory nerve coaptation, longer time since surgery and lower flap weight are identified as secondary factors that enhance sensory recovery. Higher age and chemotherapy negatively contribute to postoperative sensation (80).
Quantitative outcomes—alternative and non-abdominal flaps
Although comparable to conventional DIEP flaps, innervated bipedicled DIEP and SHAEP flaps have not been studied yet. It is therefore not known whether the superior sensory outcomes of DIEP and TRAM flaps apply similarly to bipedicled DIEP and SHAEP flaps.
Innervated LD flaps have been studied for autologous breast reconstruction, using branches of the thoracic intercostal nerves as a donor nerve. Postoperative sensory measurements indicate improved sensation to touch and pain in the innervated LD flaps (22).
In innervated SGAP flaps, tactile and erogenous sensation recover better compared with non-innervated SGAP flaps, according to Blondeel [1999] (81). However, these findings were not supported by results of sensory measurements. No other studies have attempted to quantify the sensory recovery of innervated SGAP flaps. Also for innervated IGAP and ScGAP flaps, no studies have been conducted yet.
Regarding thigh-based flaps, a prospective comparison of innervated versus non-innervated LTP flaps by Beugels et al. demonstrated superior tactile sensation of the innervated flaps with a follow-up more than 12 months (82). For DUG/TUG and PAP flaps, no studies have been published that provide objective sensory outcomes.
Hence, well-designed prospective studies that compare innervated and non-innervated autologous breast reconstruction are lacking for the majority of non-abdominal flaps. However, all existing evidence suggests superior sensory recovery in autologous breast reconstruction with innervated compared to non-innervated LTP, SGAP and LD flaps.
Quantitative outcomes—donor site
Few studies have evaluated the effect of sensory nerve harvest on donor site sensation. Two studies evaluated abdominal donor site sensation, but did not evaluate the effect of flap or sensory nerve harvest on this (83,84). Two other studies, however, did evaluate the effect of sensory nerve harvest for autologous breast reconstruction on the donor site sensation. In the first, Beugels et al. measured pre- and postoperative donor site sensation in innervated and non-innervated DIEP flaps and found that it was not compromised after donor nerve harvest (80). In another study, Beugels et al. performed the same evaluation of donor site sensation, but in LTP flaps. Nerve harvest in LTP flaps did not compromise postoperative donor site sensation either (82). For other donor sites, the effect of nerve harvest on postoperative sensation has not been analyzed.
Patient-reported outcomes—breast
The previous section demonstrates that sensory nerve coaptation improves sensation in reconstructed breasts. However, the appreciation of the patient is pivotal when exploring its clinical value. Patient satisfaction and quality of life after breast reconstruction can be evaluated using questionnaires. The BREAST-Q is considered the gold standard.
Temple et al. were the first to study the influence of sensory nerve coaptation in TRAM flap breast reconstruction on quality of life (85). Their findings entail significantly improved scores on six out of the eight domains of the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36); significantly improved scores on five out of six domains of the Body Image after Breast Cancer Questionnaire; and improved scores on all four domains of the Functional Assessment of Cancer Therapy-Breast scale. Given the positive results on each questionnaire, the authors conclude that sensory nerve coaptation adds positively to the patient-reported quality of life after TRAM flap breast reconstruction.
The same was established for DIEP flap breast reconstruction. Cornelissen et al. conducted a retrospective pilot study to compare quality of life expressed in BREAST-Q scores after innervated versus non-innervated DIEP flap breast reconstruction. A significant correlation between sensory nerve coaptation and the BREAST-Q score on the domain “Physical Wellbeing Chest” was demonstrated. The objective sensory measurements correlated significantly with this BREAST-Q score as well (86).
A larger and prospective follow-up study confirmed that quality of life improves in patients with innervated DIEP flap breast reconstruction. On average, patients with immediate and delayed innervated DIEP flap breast reconstruction score higher on the BREAST-Q domain “Physical Wellbeing Chest” compared with their non-innervated counterparts (9). Multiple other studies have aimed to assess sensation as a patient-reported outcome, however, in the vast majority, unspecific and non-validated measures were applied that poorly reflect qualitative appraisal of sensation in reconstructed breasts (87).
Based on the few methodologically sound studies using validated measures, sensory nerve coaptation improves patient-reported quality of life, and therefore is a clinically meaningful addition to autologous breast reconstruction.
Patient-reported outcomes—donor site
PROs related to the donor site can also be explored. Although developed for obese or post-bariatric patients, several “Appearance” scales of the BODY-Q have been used to measure donor-site related quality of life (88). These are, however, not validated for the autologous breast reconstruction population.
For abdominal flaps, the postoperative BREAST-Q reconstruction scale “Physical Wellbeing Abdomen” is validated as PRO for postoperative satisfaction with the donor site. However, it has not been used in studies concerning innervated flaps. Similar BREAST-Q reconstruction scales have not been developed for non-abdominal donor sites.
Qualitative appraisal of postoperative donor site sensation is still an unexplored territory. There are no patient-reported outcome measures available yet that cover this aspect.
Clinical implications
We aim to increase awareness among microsurgeons about the clinically added value of innervated breast reconstruction. Patients consulting for (autologous) breast reconstruction should be informed about the possibility to perform sensory nerve coaptation, to improve postoperative breast sensation. All current evidence indicates superior outcomes regarding sensory recovery and patient satisfaction, and no adverse outcomes have been reported. Hence, the benefits clearly outweigh the risks, of which there are none. Results from sensory measurements provide patients with objective counseling on the extent of sensory improvement to expect for certain reconstruction types, both delayed and immediate.
Sensory nerve coaptation is a surgical technique that any trained microsurgeon is capable of performing. It involves dissection at donor and recipient sites, followed by microscopic anastomosis, similar to vascular dissection and anastomosis. It can therefore readily be implemented in any microsurgeon’s clinical practice.
In this article’s senior author’s experience, identifying the nerves and dissecting them ease and fasten by practice. We therefore encourage microsurgeons to familiarize themselves with the technique and start clinical implementation.
In the changed landscape of healthcare, where PROs increasingly define the success of any medical procedure, tools such as the BREAST-Q are invaluable to monitor the clinically added value of new surgical techniques such as innervated breast reconstruction. It is therefore highly recommended to monitor outcomes of sensory nerve coaptation using validated PRO measures such as the BREAST-Q Sensation module.
Discussion
Microsurgical techniques have advanced rapidly over time, enabling the reconstruction of a breast that appears natural. Major advances over implant-based reconstruction are the absence of a foreign body, and that the results are permanent. The first mention of innervated flaps for breast reconstruction was in 1992 (6). Evidence for safety and efficacy remained sparse at that time, but grew gradually since. In the last decade the interest has expanded tremendously, and the body of evidence in favor of innervated autologous breast reconstruction is steadily increasing. Currently published research consistently points out that sensation recovers better and earlier in innervated flaps. Eventually, innervated flaps have a higher chance of approaching normal sensation, compared with non-innervated flaps.
Furthermore, better sensory recovery subsequently improves patient satisfaction and quality of life. Modern-era healthcare has evolved to become increasingly patient-centered. Patient-reported outcomes such as QoL are therefore valued highly and often steer changes in clinical practice. The positive effect of improved breast sensation on PROs is therefore essential for it to be widely implemented. It deserves recommendation to routinely administer the BREAST-Q for monitoring of these outcomes in clinical practice. A critical note, however, is that patient-reported sensation is not yet explored, both for the breast as well as the donor site. The novel BREAST-Q Sensation module has potential to more reliably determine the value of sensory nerve coaptation in autologous breast reconstruction (39). This module facilitates improved reflection of patient satisfaction related to sensation in the reconstructed breast, and should therefore be encouraged to use in novel clinical research. For the donor site sensation, no QoL measures have been developed, however, current research does not indicate that donor site sensation is influenced by sensory nerve harvest (78,82). Secondly, satisfaction and QoL may be improved by refining preoperative patient education. As stated previously, preoperative expectations considerably affect postoperative satisfaction. Therefore, educating patients about changes in sensation is advisable as it will likely improve patient satisfaction. Improving patient satisfaction and quality of life is the most important goal of breast reconstruction; this is therefore the most important argument to educate patients about postoperative breast sensation and endeavoring to improve it.
Besides outcomes related to efficacy and quality of life, safety of novel surgical techniques also needs to be established. It deserves highlighted notice that no major, nor minor adverse outcomes related to the nerve coaptation have been reported. Furthermore, when coapting the nerves within the same microsurgical field as the vascular anastomoses, operative time is not significantly prolonged. Hence, there are no known risks or disadvantages related to sensory nerve coaptation.
The unanimously demonstrated super outcomes of innervated versus non-innervated flaps for breast reconstruction, combined with the absence of risks and disadvantages, strongly advocates implementation of this technique in clinical practice.
The future of innervated flaps for breast reconstruction
Although current evidence speaks in favor of innervated breast reconstruction, some caution is still required with drawing definite conclusions, as double-blinded randomized trials are still lacking and the level of evidence is therefore suboptimal. Systematic reviews on this subject all performed quality assessment, showing varying levels of quality of the included studies. Furthermore, large methodological inconsistencies across studies are unanimously criticized. This includes testing modality, time since surgery, tested areas of the breast, surgical techniques, and also outcome reporting and analysis (10-14,47). Since the concept of sensation is multifaceted, it remains challenging to address all different modalities of sensation in a uniform manner.
Secondly, sensation in non-abdominal flaps need to be explored further. As the proportion of patients that opt for breast reconstruction after mastectomy increases over time, the demand for autologous breast reconstruction may further increase (89). Depending on physical appearance or medical history, abdominal flaps are suboptimal for specific patients. For this population, better understanding of sensation in non-abdominal flaps and the possibilities for innervation may aid clinical decision-making.
Additionally, the surgical approach may be refined and optimized in the future. For example, by exploring the role of targeted nipple-areolar complex (NAC) re-innervation. Peled and Peled first proposed the distal, subareolar nerve ending as a target for reinnervation (42). In implant-based breast reconstruction this dissected subareolar nerve can be coapted to a proximally transected intercostal nerve, aided by a nerve (allo)graft. This technique can be applied to autologous reconstruction by tunneling the nerve through the flap (90). If no nerve stump can be identified, the nerve graft can be sutured to the dermis of the NAC instead. Hence, this technique may be valuable in breast reconstruction after nipple-sparing mastectomy.
Another promising future perspective is proposed by Chen et al., as they apply their robotic microsurgical expertise to peripheral nerve surgery (91). Specific to autologous breast reconstruction, they described a method to robotically harvest the fourth intercostal nerve. Consequently, the harvested nerve can be used as autograft to elongate the thoracic recipient nerve. This elegant technique combines the advantages of autografts, such as low costs and the absence of alloplastic grafts, with the advantages of a minimally invasive robotic approach.
One additional aspect that cannot be ignored is that regardless of sensory nerve coaptation, postoperative sensation does not return to normal. First of all, this implies the need to further optimize the surgical technique. The use of nerve conduits or grafts, coapting multiple nerves, and using different recipient nerves are some aspects that need elucidating in order to define the optimal surgical strategy. But secondly, the inability to regain normal sensation also implies that fundamental processes in sensory recovery are not yet fully understood. Increasing knowledge about the fundamental principles of (recovery of) sensation and the inclusion of a multidisciplinary team of scientific collaborators from different fields, is therefore a critical next step.
Aside from continuation of both clinical and fundamental research on this subject, we encourage wide implementation of innervated breast reconstruction in clinical practice. Sensory nerve coaptation requires merely basic microsurgical skills. Every trained microsurgeon is adequately skilled to perform sensory nerve coaptation. The most challenging aspect is recognition of the nerves. In our experience, the learning curve for successful nerve coaptation lies predominantly in training of the visual search skills for identification of the nerves. The success rate naturally improves when the intention to coapt nerves grows.
Conclusion
Sensory nerve coaptation is a valuable addition to autologous breast reconstruction. All current literature unambiguously demonstrates favorable outcomes of innervated over non-innervated breast reconstruction; as indicated by earlier return of sensation and higher chances of approaching normal sensation. It improves functional and psychosocial outcomes, as well as quality of life. Due to newly developed patient-reported outcome measures, such as the BREAST-Q sensation module, sensation-related quality of life can be measured more specifically in the future. Importantly, there are no associated risks or disadvantages, and operative time is only marginally increased. Therefore, we argue strongly that sensory nerve coaptation is worthwhile to implement in standard clinical practice.
Acknowledgments
The authors wish to thank Greet Mommen, medical illustrator of the Department of Anatomy and Embryology at Maastricht University, for preparing the illustrations; and Arno Lataster for his valuable expertise and insights regarding the anatomical parts of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ketan M. Patel and Ara A. Salibian) for the series “Advances in Microsurgical Breast Reconstruction” published in Gland Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-40/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-40/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-40/coif). The series “Advances in Microsurgical Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rozen WM, Rajkomar AK, Anavekar NS, et al. Post-mastectomy breast reconstruction: a history in evolution. Clin Breast Cancer 2009;9:145-54. [Crossref] [PubMed]
- Homsy A, Rüegg E, Montandon D, et al. Breast Reconstruction: A Century of Controversies and Progress. Ann Plast Surg 2018;80:457-63. [Crossref] [PubMed]
- Rabin RC. After mastectomies, an unexpected blow: numb new breasts. New York Times. 2017 January 29, 2017. Available online: https://www.nytimes.com/2017/01/29/well/live/after-mastectomies-an-unexpected-blow-numb-new-breasts.html
- Cornelissen AJM, Tuinder SMH, Heuts EM, et al. What does a breast feel like? A qualitative study among healthy women. BMC Womens Health 2018;18:82. [Crossref] [PubMed]
- Enajat M, Rozen WM, Audolfsson T, et al. Thermal injuries in the insensate deep inferior epigastric artery perforator flap: case series and literature review on mechanisms of injury. Microsurgery 2009;29:214-7. [Crossref] [PubMed]
- Slezak S, McGibbon B, Dellon AL. The sensational transverse rectus abdominis musculocutaneous (TRAM) flap: return of sensibility after TRAM breast reconstruction. Ann Plast Surg 1992;28:210-7. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Spiegel AJ, Menn ZK, Eldor L, et al. Breast Reinnervation: DIEP Neurotization Using the Third Anterior Intercostal Nerve. Plast Reconstr Surg Glob Open 2013;1:e72. [Crossref] [PubMed]
- Bijkerk E, Beugels J, van Kuijk SMJ, et al. Clinical Relevance of Sensory Nerve Coaptation in DIEP Flap Breast Reconstruction Evaluated Using the BREAST-Q. Plast Reconstr Surg 2022;150:959e-69e. [Crossref] [PubMed]
- Beugels J, Cornelissen AJM, Spiegel AJ, et al. Sensory recovery of the breast after innervated and non-innervated autologous breast reconstructions: A systematic review. J Plast Reconstr Aesthet Surg 2017;70:1229-41. [Crossref] [PubMed]
- Weissler JM, Koltz PF, Carney MJ, et al. Sifting through the Evidence: A Comprehensive Review and Analysis of Neurotization in Breast Reconstruction. Plast Reconstr Surg 2018;141:550-65. [Crossref] [PubMed]
- Chou J, Hyland CJ, Kaufman Goldberg T, et al. Is nerve coaptation associated with improved sensation after microvascular breast reconstruction? A systematic review. Microsurgery 2023;43:522-8. [Crossref] [PubMed]
- Shiah E, Laikhter E, Comer CD, et al. Neurotization in Innervated Breast Reconstruction: A Systematic Review of Techniques and Outcomes. J Plast Reconstr Aesthet Surg 2022;75:2890-913. [Crossref] [PubMed]
- Abbas F, Klomparens K, Simman R. Functional and Psychosocial Outcomes following Innervated Breast Reconstruction: A Systematic Review. Plast Reconstr Surg Glob Open 2022;10:e4559. [Crossref] [PubMed]
- Chin KJ, Versyck B, Pawa A. Ultrasound-guided fascial plane blocks of the chest wall: a state-of-the-art review. Anaesthesia 2021;76:110-26. [Crossref] [PubMed]
- Woodworth GE, Ivie RMJ, Nelson SM, et al. Perioperative Breast Analgesia: A Qualitative Review of Anatomy and Regional Techniques. Reg Anesth Pain Med 2017;42:609-31. [Crossref] [PubMed]
- Moore K, Dalley A, Agur A. Clinically oriented anatomy (6th ed.). Philadelphia: Lippincott Williams & Wilkins; 2010:98-9.
- Smeele HP, Bijkerk E, van Kuijk SMJ, et al. Innervation of the Female Breast and Nipple: A Systematic Review and Meta-Analysis of Anatomical Dissection Studies. Plast Reconstr Surg 2022;150:243-55. [Crossref] [PubMed]
- Bijkerk E, Cornelissen AJM, Sommer M, et al. Intercostal nerve block of the anterior cutaneous branches and the sensibility of the female breast. Clin Anat 2020;33:1025-32. [Crossref] [PubMed]
- Mol FMU, Lataster A, Scheltinga M, Roumen R. Anatomy of abdominal anterior cutaneous intercostal nerves with respect to the pathophysiology of anterior cutaneous nerve entrapment syndrome (ACNES): A case study. Translational Research in Anatomy 2017;8-9:6-10. [Crossref]
- Cakmakoglu C, Knackstedt R, Gatherwright J, et al. Determining the precise anatomic location of the sensory nerves to the abdominal wall: Optimizing autologous innervation of abdominally based free flaps. J Plast Reconstr Aesthet Surg 2021;74:641-3. [Crossref] [PubMed]
- Yano K, Hosokawa K, Takagi S, et al. Breast reconstruction using the sensate latissimus dorsi musculocutaneous flap. Plast Reconstr Surg 2002;109:1897-902; discussion 1903. [Crossref] [PubMed]
- Rozen WM, Tran TM, Ashton MW, et al. Refining the course of the thoracolumbar nerves: a new understanding of the innervation of the anterior abdominal wall. Clin Anat 2008;21:325-33. [Crossref] [PubMed]
- Knackstedt R, Gatherwright J, Drake R, et al. Anatomic location of sensory nerves to the superior and inferior gluteal artery perforators flap: Novel option for sensate autologous tissue reconstruction. J Plast Reconstr Aesthet Surg 2019;72:1576-606. [Crossref] [PubMed]
- Gatherwright J, Knackstedt R, Djohan R. Anatomic Targets for Breast Reconstruction Neurotization: Past Results and Future Possibilities. Ann Plast Surg 2019;82:207-12. [Crossref] [PubMed]
- Knackstedt R, Djohan R, Gatherwright J. Anatomic location of a sensory nerve to the lateral thigh flap: A novel option for sensate autologous tissue reconstruction. J Plast Reconstr Aesthet Surg 2019;72:513-27. [Crossref] [PubMed]
- Purves D, Augustine GJ, Fitzpatrick D, et al. Mechanoreceptors Specialized to Receive Tactile Information. Neuroscience 2nd Edition. Sunderland (MA), 2001.
- Courtiss EH, Goldwyn RM. Breast sensation before and after plastic surgery. Plast Reconstr Surg 1976;58:1-13. [Crossref] [PubMed]
- Tairych GV, Kuzbari R, Rigel S, et al. Normal cutaneous sensibility of the breast. Plast Reconstr Surg 1998;102:701-4. [Crossref] [PubMed]
- Terzis JK, Vincent MP, Wilkins LM, et al. Breast sensibility: a neurophysiological appraisal in the normal breast. Ann Plast Surg 1987;19:318-22. [Crossref] [PubMed]
- Longo B, Campanale A, Santanelli di Pompeo F. Nipple-areola complex cutaneous sensitivity: a systematic approach to classification and breast volume. J Plast Reconstr Aesthet Surg 2014;67:1630-6. [Crossref] [PubMed]
- Kasielska-Trojan A, Szulia A, Zawadzki T, et al. The Assessment of Nipple Areola Complex Sensation with Semmes-Weinstein Monofilaments-Normative Values and Its Covariates. Diagnostics (Basel) 2021;11:2145. [Crossref] [PubMed]
- Penfield W, Boldrey E. Somatic Motor and Sensory Representation in the Cerebral Cortex of Man as Studied by Electrical Stimulation. Brain 1937;60:389-443. [Crossref]
- Penfield W, Rasmussen T. The cerebral cortex of man; a clinical study of localization of function. New York: Macmillan; 1950:xv, 248 pp.
- Di Noto PM, Newman L, Wall S, et al. The hermunculus: what is known about the representation of the female body in the brain? Cereb Cortex 2013;23:1005-13. [Crossref] [PubMed]
- Rothemund Y, Schaefer M, Grüsser SM, et al. Localization of the human female breast in primary somatosensory cortex. Exp Brain Res 2005;164:357-64. [Crossref] [PubMed]
- Beugels J, van den Hurk J, Peters JC, et al. Somatotopic mapping of the human breast using 7 T functional MRI. Neuroimage 2020;204:116201. [Crossref] [PubMed]
- Cornelissen AJM, Beugels J, Lataster A, et al. Comparing the sensation of common donor site regions for autologous breast reconstruction to that of a healthy breast. J Plast Reconstr Aesthet Surg 2018;71:327-35. [Crossref] [PubMed]
- Tsangaris E, Klassen AF, Kaur MN, et al. Development and Psychometric Validation of the BREAST-Q Sensation Module for Women Undergoing Post-Mastectomy Breast Reconstruction. Ann Surg Oncol 2021;28:7842-53. [Crossref] [PubMed]
- Knackstedt R, Gatherwright J, Cakmakoglu C, et al. Predictable Location of Breast Sensory Nerves for Breast Reinnervation. Plast Reconstr Surg 2019;143:393-6. [Crossref] [PubMed]
- Hamilton KL, Kania KE, Spiegel AJ. Post-mastectomy sensory recovery and restoration. Gland Surg 2021;10:494-7. [Crossref] [PubMed]
- Peled AW, Peled ZM. Nerve Preservation and Allografting for Sensory Innervation Following Immediate Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2332. [Crossref] [PubMed]
- Bijkerk E, van Kuijk SMJ, Beugels J, et al. Breast sensibility after mastectomy and implant-based breast reconstruction. Breast Cancer Res Treat 2019;175:369-78. [Crossref] [PubMed]
- Lagergren J, Wickman M, Hansson P. Sensation Following Immediate Breast Reconstruction with Implants. Breast J 2010;16:633-8. [Crossref] [PubMed]
- Mohanna PN, Raveendran SS, Ross DA, et al. Thermal injuries to autologous breast reconstructions and their donor sites--literature review and report of six cases. J Plast Reconstr Aesthet Surg 2010;63:e255-60. [Crossref] [PubMed]
- Magarakis M, Venkat R, Dellon AL, et al. Pilot study of breast sensation after breast reconstruction: evaluating the effects of radiation therapy and perforator flap neurotization on sensory recovery. Microsurgery 2013;33:421-31. [Crossref] [PubMed]
- Shridharani SM, Magarakis M, Stapleton SM, et al. Breast sensation after breast reconstruction: a systematic review. J Reconstr Microsurg 2010;26:303-10. [Crossref] [PubMed]
- de Boer M, van der Hulst R, Slatman J. The Surprise of a Breast Reconstruction: A Longitudinal Phenomenological Study to Women’s Expectations About Reconstructive Surgery. Human Studies 2015;38:409-30. [Crossref]
- Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist 1985;40:1189-202. [Crossref]
- Thompson AG, Suñol R. Expectations as determinants of patient satisfaction: concepts, theory and evidence. Int J Qual Health Care 1995;7:127-41. [Crossref] [PubMed]
- Pusic AL, Klassen AF, Snell L, et al. Measuring and managing patient expectations for breast reconstruction: impact on quality of life and patient satisfaction. Expert Rev Pharmacoecon Outcomes Res 2012;12:149-58. [Crossref] [PubMed]
- Spiegel AJ, Salazar-Reyes H, Izaddoost S, et al. A novel method for neurotization of deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg 2009;123:29e-30e. [Crossref] [PubMed]
- Mohan AT, Suchyta M, Vyas KS, et al. A Cadaveric Anatomical and Histological Study of Recipient Intercostal Nerve Selection for Sensory Reinnervation in Autologous Breast Reconstruction. J Reconstr Microsurg 2021;37:136-42. [Crossref] [PubMed]
- Bassilios Habre S, Bond G, Jing XL, et al. The Surgical Management of Nerve Gaps: Present and Future. Ann Plast Surg 2018;80:252-61. [Crossref] [PubMed]
- Safa B, Buncke G. Autograft Substitutes: Conduits and Processed Nerve Allografts. Hand Clin 2016;32:127-40. [Crossref] [PubMed]
- Momeni A, Meyer S, Shefren K, et al. Flap Neurotization in Breast Reconstruction with Nerve Allografts: 1-year Clinical Outcomes. Plast Reconstr Surg Glob Open 2021;9:e3328. [Crossref] [PubMed]
- Gao Y, Wang YL, Kong D, et al. Nerve autografts and tissue-engineered materials for the repair of peripheral nerve injuries: a 5-year bibliometric analysis. Neural Regen Res 2015;10:1003-8. [Crossref] [PubMed]
- Sabongi RG, Fernandes M, Dos Santos JB. Peripheral nerve regeneration with conduits: use of vein tubes. Neural Regen Res 2015;10:529-33. [Crossref] [PubMed]
- Djohan R, Scomacao I, Duraes EFR, et al. Sensory Restoration in Abdominally Based Free Flaps for Breast Reconstruction Using Nerve Allograft. Plast Reconstr Surg 2023;151:25-33. [Crossref] [PubMed]
- Beugels J, Vasile JV, Tuinder SMH, et al. The Stacked Hemiabdominal Extended Perforator Flap for Autologous Breast Reconstruction. Plast Reconstr Surg 2018;142:1424-34. [Crossref] [PubMed]
- Stillaert FBJL, Opsomer D, Blondeel PN, et al. The Lumbar Artery Perforator Flap in Breast Reconstruction. Plast Reconstr Surg 2023;151:41-4. [Crossref] [PubMed]
- Netter FH. Atlas of Human Anatomy, 7th edition. Philadelphia, PA: Elsevier; 2018.
- Granzow JW, Levine JL, Chiu ES, et al. Breast reconstruction with gluteal artery perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:614-21. [Crossref] [PubMed]
- LoTempio MM, Allen RJ. Breast reconstruction with SGAP and IGAP flaps. Plast Reconstr Surg 2010;126:393-401. [Crossref] [PubMed]
- Tuinder S, Chen CM, Massey MF, et al. Introducing the septocutaneous gluteal artery perforator flap: a simplified approach to microsurgical breast reconstruction. Plast Reconstr Surg 2011;127:489-95. [Crossref] [PubMed]
- Dayan E, Smith ML, Sultan M, et al. The Diagonal Upper Gracilis (DUG) Flap: A Safe and Improved Alternative to the TUG Flap. Plastic and Reconstructive Surgery 2013;132:33-4. [Crossref]
- Gatherwright J, Knackstedt R, Kurlander D, et al. Anatomic location of a sensory nerve to the transverse upper gracilis (TUG) flap: A novel option for sensate autologous tissue reconstruction. J Plast Reconstr Aesthet Surg 2019;72:137-71. [Crossref] [PubMed]
- Knackstedt R, Gatherwright J, Djohan R. Anatomic location of a sensory nerve to the profunda artery perforator (PAP) flap: A novel option for sensate autologous tissue reconstruction. J Plast Reconstr Aesthet Surg 2019;72:2064-94. [Crossref] [PubMed]
- Yano T, Karakawa R, Yoshimatsu H, et al. The Feasibility of Harvesting an Innervated Profunda Artery Perforator Flap for Breast Reconstruction. Plast Reconstr Surg Glob Open 2020;8:e3160. [Crossref] [PubMed]
- Doncatto L, Hochberg J, Caleffi M. Breast Reconstruction with Sensitive TRAM Flap Reinnervation. The Breast Journal. 1997;3:345-9. [Crossref]
- Yano K, Matsuo Y, Hosokawa K. Breast reconstruction by means of innervated rectus abdominis myocutaneous flap. Plast Reconstr Surg 1998;102:1452-60. [Crossref] [PubMed]
- Isenberg JS. Sense and sensibility: breast reconstruction with innervated TRAM flaps. J Reconstr Microsurg 2002;18:23-8. [Crossref] [PubMed]
- Yap LH, Whiten SC, Forster A, et al. Sensory recovery in the sensate free transverse rectus abdominis myocutaneous flap. Plast Reconstr Surg 2005;115:1280-8. [Crossref] [PubMed]
- Temple CL, Tse R, Bettger-Hahn M, et al. Sensibility following innervated free TRAM flap for breast reconstruction. Plast Reconstr Surg 2006;117:2119-27; discussion 2128-30. [Crossref] [PubMed]
- Puonti HK, Jääskeläinen SK, Hallikainen HK, et al. A new approach to microneurovascular TRAM-flap breast reconstruction--a pilot study. J Plast Reconstr Aesthet Surg 2011;64:346-52. [Crossref] [PubMed]
- Puonti HK, Jääskeläinen SK, Hallikainen HK, et al. Improved sensory recovery with a novel dual neurorrhaphy technique for breast reconstruction with free muscle sparing TRAM flap technique. Microsurgery 2017;37:21-8. [Crossref] [PubMed]
- Blondeel PN, Demuynck M, Mete D, et al. Sensory nerve repair in perforator flaps for autologous breast reconstruction: sensational or senseless? Br J Plast Surg 1999;52:37-44. [Crossref] [PubMed]
- Beugels J, Cornelissen AJM, van Kuijk SMJ, et al. Sensory Recovery of the Breast following Innervated and Noninnervated DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2019;144:178e-88e. [Crossref] [PubMed]
- Bijkerk E, van Kuijk SMJ, Lataster A, et al. Breast sensibility in bilateral autologous breast reconstruction with unilateral sensory nerve coaptation. Breast Cancer Res Treat 2020;181:599-610. [Crossref] [PubMed]
- Beugels J, Bijkerk E, Lataster A, et al. Nerve Coaptation Improves the Sensory Recovery of the Breast in DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2021;148:273-84. [Crossref] [PubMed]
- Blondeel PN. The sensate free superior gluteal artery perforator (S-GAP) flap: a valuable alternative in autologous breast reconstruction. Br J Plast Surg 1999;52:185-93. [Crossref] [PubMed]
- Beugels J, van Kuijk SMJ, Lataster A, et al. Sensory Recovery of the Breast following Innervated and Noninnervated Lateral Thigh Perforator Flap Breast Reconstruction. Plast Reconstr Surg 2021;147:281-92. [Crossref] [PubMed]
- Stromps JP, Bozkurt A, Grieb G, et al. Spontaneous Reinnervation of Deep Inferior Epigastric Perforator Flaps after Delayed Breast Reconstruction. J Reconstr Microsurg 2016;32:169-77. [PubMed]
- Santanelli F, Longo B, Angelini M, et al. Prospective computerized analyses of sensibility in breast reconstruction with non-reinnervated DIEP flap. Plast Reconstr Surg 2011;127:1790-5. [Crossref] [PubMed]
- Temple CLF, Ross DC, Kim S, et al. Sensibility following innervated free TRAM flap for breast reconstruction: Part II. Innervation improves patient-rated quality of life. Plast Reconstr Surg 2009;124:1419-25. [Crossref] [PubMed]
- Cornelissen AJM, Beugels J, van Kuijk SMJ, et al. Sensation of the autologous reconstructed breast improves quality of life: a pilot study. Breast Cancer Res Treat 2018;167:687-95. [Crossref] [PubMed]
- Smeele HP, Dijkstra RCH, Kimman ML, et al. Patient-Reported Outcome Measures Used for Assessing Breast Sensation after Mastectomy: Not Fit for Purpose. Patient 2022;15:435-44. [Crossref] [PubMed]
- Miseré RM, van Kuijk SM, Claassens EL, et al. Breast-related and body-related quality of life following autologous breast reconstruction is superior to implant-based breast reconstruction - A long-term follow-up study. Breast 2021;59:176-82. [Crossref] [PubMed]
- Ilonzo N, Tsang A, Tsantes S, et al. Breast reconstruction after mastectomy: A ten-year analysis of trends and immediate postoperative outcomes. Breast 2017;32:7-12. [Crossref] [PubMed]
- Tevlin R, Brazio P, Tran N, et al. Immediate targeted nipple-areolar complex re-innervation: Improving outcomes in immediate autologous breast reconstruction. J Plast Reconstr Aesthet Surg 2021;74:1503-7. [Crossref] [PubMed]
- Chen LW, Goh M, Goh R, et al. Robotic-Assisted Peripheral Nerve Surgery: A Systematic Review. J Reconstr Microsurg 2021;37:503-13. [Crossref] [PubMed]


