
Chinese guidelines on intraoperative neuromonitoring in thyroid and parathyroid surgery (2023 edition)
Highlight box
Key recommendations
• Setting appropriate event thresholds can help improve the effectiveness of early warning for nerve injury.
• Thyroid cartilage needle electrodes can also be used as the recording electrodes.
• The monitoring point comparison method can be used to indicate the presence of an NRLN.
• Stimulating electrodes can be placed in the innervation areas, thus locating, identifying, and protecting these motor nerves.
What was recommended and what is new?
• In previous version of Chinese guidelines on IONM in thyroid and parathyroid surgery, only point comparison method was recommended as the way to recognize NRLN.
• In this version, the monitoring point comparison method and latency assessment method can both recognize NRLN.
What is the implication, and what should change now?
• Standardized solutions for various troubleshooting are included in the guidelines.
• The new recommendations in the guidelines are more favorable for the clinical application of IONM.
Introduction
Protecting the laryngeal nerves has long been a priority and challenge in thyroid and parathyroid surgeries. As an auxiliary tool, intraoperative neuromonitoring (IONM) has played an active role in the protection of nerve function. In order to help clinicians understand, standardize and reasonably apply IONM techniques, the Chinese Thyroid Association, College of Surgeons, Chinese Medical Doctor Association, and the Chinese Research Hospital Association Thyroid Disease Committee, with the joint efforts of some several other societies and experts, published the “Clinical guidelines on intraoperative neuromonitoring in thyroid and parathyroid surgeries (Chinese edition)”, “Expert consensus on protection and monitoring of the external branch of the superior laryngeal nerve during thyroid and parathyroid surgeries (2017 version)”, and “Expert consensus on neurophysiological monitoring in robotic thyroid and parathyroid surgeries (2019 version)” in 2013, 2017, and 2019, respectively (1-3), which received wide attention and recognition from the medical community. We present this article in accordance with the RIGHT reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-284/rc).
Methods
All efforts have been made to ensure that these new guidelines are practical, systematic, and based on well-recognized cutting-edge evidence. Using the three previous guidelines as well as new preclinical and clinical evidence from China and other countries, the expert panel summarized the current accepted or near-accepted opinions as the recommendations. In addition, the recommendation grades and evidence levels are presented in accordance with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) (Table 1) (4). The first draft was completed by the writing team and then revised after several rounds of discussions via email or meetings. The finalized version includes 42 recommendations, which may inform and guide our peers in their clinical practice. These guidelines apply to all types of open, endoscopic, and robotic thyroid and parathyroid surgeries.
Table 1
| Category | Definition | Notes |
|---|---|---|
| Grade of recommendation | A (strong recommendation) | Recommendations, for or against a particular management approach, are usually applicable to most patients |
| Benefits clearly outweigh the risk and burdens, or vice versa | ||
| B (weak recommendations) | Recommendations, for or against a particular management approach, may be conditional upon patients’ conditions and preferences | |
| The relationship between benefits and risks/burdens is more balanced or uncertain | ||
| C (no recommendation) | There is insufficient evidence to recommend for or against a particular management approach | |
| Level of evidence | A (high quality) | Evidence at a low risk of bias (e.g., consistent results from high-quality randomized trials) that can be applied directly to recommendations |
| B (intermediate quality) | Study limitations, inconsistent results, and indirect evidence | |
| C (low quality) | Case analysis or nonsystematic clinical observations, with inadequate evidence |
GRADE, Grading of Recommendations Assessment, Development and Evaluation.
IONM: advances and applications
Over the past half-century, rapid advances have been made in IONM for thyroid surgeries in terms of techniques, application ideas and methods, monitoring systems and equipment, and clinical application scenarios. More specifically, the monitoring parameters have evolved from non-electromyographic (non-EMG) to EMG forms, facilitating the visualization and quantification of neurological function and making functional assessment more accurate. The monitoring methods have also evolved from intermittent-IONM (I-IONM), which involves use of a handheld probe that offers intraoperative current stimulation on demand, to continuous-IONM (C-IONM), which involves use of implanted electrodes to allow the periodic release of current stimulation and thus real-time neurological function monitoring throughout the operation. The nerves monitored have expanded from the recurrent laryngeal nerve (RLN) and vagus nerve (VN) to the external branch of the superior laryngeal nerve (EBSLN) and other motor nerves in the neck. Finally, the application scenarios have also included endoscopic and robotic surgeries, as well as conventional open surgeries. In combining both functional and anatomic techniques, IONM not only promotes the transition of intraoperative nerve function protection from the “experience-based” to the “precision” concept, but also helps to improve the quality and efficiency of thyroid surgeries, thus promoting the rapid development of thyroid surgery.
The clinical application of IONM in thyroid surgery is valuable as it can (I) efficiently locate and identify nerve courses, identify nerve branches, anatomical variants and indistinguishable tissues, and avoid excessive anatomical dissection or mobilization (5); (II) provide real-time intraoperative feedback on neurophysiological function to alert surgeons of the potential risks of nerve injury or high-risk operations and thus improve surgical safety and thoroughness (6); and (III) assist in the mechanism analysis and severity assessment of nerve injury and enable the prompt removal of the cause of injury to correct any reversible damage (7). IONM can guide the development of individualized surgical strategies to remove the risk of serious postoperative complications caused by simultaneous bilateral nerve injury (7,8). Since IONM has multiple advantages in nerve identification, functional protection, and injury prevention and control, in theory, it can feasibly help protect the motor nerves in all types of thyroid and parathyroid surgeries, especially in surgeries with high risk of nerve injury; in endoscopic- or robotic-assisted surgeries with relatively small operating space; and in cases where the patients have high requirements for voice preservation (9).
Despite its technical advantages, IONM for thyroid surgery also has some limitations. The accuracy of intraoperative EMG loss of signal (LOS) in predicting postoperative vocal cord paralysis (VCP) is highly variable (10% to 90%) (10), and the frequency of equipment-related problems is approximately 4% to 23% during the monitoring (11).
Recommendation 1. IONM in thyroid and parathyroid surgeries enables both functional and anatomic monitoring and is an effective adjunct for intraoperative nerve protection. (Grade of recommendation: A; level of evidence: A).
Recommendation 2. IONM techniques assisting in neurological function protection are feasible for procedures with a higher risk of nerve injury, for endoscopic- or robotic-assisted surgeries with relatively small operating space, and in cases where the patients have high voice requirements. (Grade of recommendation: A; level of evidence: B).
IONM principles, parameters, and equipment
Basic principles of IONM
The principle of IONM is as follows: a stimulating electrode releases electric current to the nerve; the motor nerves can form nerve impulses and transmit them to the innervated muscle, inducing muscle contraction and generating EMG signals, which can be received by the recording electrodes in the muscle and transmitted back to the nerve monitor for amplification and processing, forming EMG signals and cueing sound, which can indicate the functional status of the nerve (12).
Recommendation 3. IONM should be used in the form of EMG to reflect nerve function objectively, accurately, and quantitatively. (Grade of recommendation: A; level of evidence: B).
Interpretation of EMG parameters
The measurement, analysis, and recording of EMG signals are clinically important for assessing neurological function. The main EMG parameters include amplitude, latency, area under the waveform (AUW), and term (or period).
Amplitude: amplitude, measured in µV, refers to the difference between the peak voltage and trough voltage of an EMG waveform. The amplitude represents the sum of motor unit action potential (MUAP) of muscles and is related to the number of muscle fibers involved in the concurrent depolarization. The changes in intraoperative amplitude directly indicate the change in nerve function. Thus, the amplitude is the most important indicator of nerve function (13).
Latency: latency, measured in ms, refers to the time delay between the initiation of electrical stimulation and the moment when the first waveform begins to leave the baseline. The length of latency is related to the rate at which nerve depolarization occurs after electrical stimulation, and the distance between the stimulation site and the effector muscle directly determines the latency value. Therefore, latency variation can assist in discriminating nerves, especially in the determination of nonrecurrent laryngeal nerves (NRLNs) versus RLNs (13).
AUW: AUW, measured in pt, refers to the area of a closed figure enclosed by the EMG waveform and the baseline. AUW is related to the number of muscle fibers involved in muscle action potentials and is a more sensitive feature indicator of changes in motor nerve function. It is suitable for monitoring nerves (such as the EBSLN) with low amplitude and short latency and term (14).
Term: term, measured in ms, refers to the time taken by the EMG waveform to leave and return to the baseline. Term reflects the depolarization time of the effector muscle and is related to the degree of synchronous excitation of nerve fibers. It can be used to distinguish the different types of effector muscles and is mostly applied in EMG signal analysis (13).
Recommendation 4. The amplitude and latency of EMG are important indicators of nerve function and should be carefully observed during surgery. (Grade of recommendation: A; level of evidence: A).
Operating room layout for IONM
A typical IONM system includes a host, stimulation end device, recording end device, and connection components.
Host
The host is a core piece of equipment in the IONM system and has multiple functions, including release of electrical stimulation, EMG signal processing, information storage, and display. Some of the parameters that need to be configured in its interface during use are described below.
Event threshold: the event threshold refers to the lowest amplitude value that can induce a response from the system. The initial event thresholds may differ and are typically low in different monitor models, which are designed only to shield against interferences. They can be configured according to the actual needs during surgery so as to increase their performance in alerting nerve injury events (15).
Stimulation current intensity: stimulation current intensity refers to the intensity of the current released at the stimulation end that acts on the nerve. Threshold stimulus refers to a current intensity that triggers the smallest recognizable EMG activity to occur in a nerve. As the suprathreshold stimulus is increased to the maximum intensity, all nerve fibers are depolarized and the maximum amplitude value of EMG evoked. Further increasing the current intensity does not result in higher amplitudes but may depolarize more tissues around the stimulation probe. Therefore, high current intensities can be used to increase sensitivity when the initial localization/identification of nerves is performed; in contrast, lower current intensities can be used to increase specificity when the fine manipulation of nerves is performed (13).
Stimulation current frequency: stimulation current frequency refers to the frequency of stimulation current released in a pulsatile manner. Usually, the current frequency system of I-IONM is preset to 4 Hz, which means that the current is released 4 times every 1 second. Therefore, constant probe-to-tissue contact should be maintained when the tissue is being probed.
Recommendation 5. All monitors have their preset event thresholds. Setting appropriate event thresholds can help improve the effectiveness of early warning for nerve injury. (Grade of recommendation: A; level of evidence: B).
Stimulation end devices
Stimulation end devices are mainly the stimulation electrodes that release current, and they can be classified as unipolar, bipolar, or multipolar electrodes. Unipolar electrodes have diffuse current distribution and are more commonly used in clinical setting to locate nerve courses through the adjustment of current intensities. Bipolar or multipolar electrodes have more concentrated current distribution and higher precision and are therefore more suitable for precise nerve localization than they are for nerve mapping (16).
Currently, the commonly used stimulation electrodes include probe electrodes, integrated electrodes, and continuous monitoring electrodes.
Unipolar ball-tipped probes are more commonly used in I-IONM, as they cause less tissue damage than flat-tipped probes. Hook- and clip-type probes are also optional for endoscopic- and robotic-assisted surgeries, as they can increase the surgical convenience and stability (3).
Integrated electrodes are stimulation electrodes that have been integrated into surgical instruments or energy instruments (e.g., probing forceps and probing scissors with functions of both electrical stimulation and anatomical dissection) (17). Alternately, the stimulation end can be connected to the corresponding surgical instruments (e.g., separation forceps, bipolar electrocoagulation device, and unipolar electrocoagulation device) to synchronize surgical operations with nerve mapping. At present, these instruments are more frequently used in endoscopic- and robotic-assisted surgeries (3,18).
During C-IONM, various types of VN continuous monitoring electrodes are used, each with its own characteristics and advantages in terms of shape, size, electrode type, and contact form with the VN trunk. These devices can be selected in accordance to the demands of the situation to ensure that the electrodes are not easily dislodged or stuck on the nerve.
Recommendation 6. Although unipolar probes are more commonly used in I-IONM, other electrodes can also be used as appropriate. (Grade of recommendation: A; level of evidence: B).
Recommendation 7. During C-IONM, the proper types of continuous VN stimulation electrodes should be used to avoid nerve injury caused by electrode dislodgement or compression. (Grade of recommendation: A; level of evidence: B).
Recommendation 8. Integrated stimulation electrodes can be used in endoscopic- and robotic-assisted surgeries to synchronize surgical operations with nerve mapping. (Grade of recommendation: B; level of evidence: C).
Recording end devices
Currently, the commonly used recording end devices are mainly monitoring catheter surface electrodes and thyroid cartilage needle electrodes.
Monitoring catheter surface electrodes record EMG signals generated by vocal folds through direct contact between surface electrodes and vocal folds. These devices can be roughly divided into two types: monitoring catheters with surface electrodes and patch electrodes that can be attached to the surface of conventional monitoring catheters. This type of monitoring catheter is simple and safe and is most commonly used in clinical practice (19). However, good contact between the surface electrodes and the bilateral vocal cords is required; in addition, the monitoring performance may be impacted if the monitoring catheter is rotated or if the placement is too deep or too shallow (Figure 1).
Thyroid cartilage needle electrodes can obtain EMG signals more directly by placing the needle recording electrodes in the lower middle area of the anterior horn of the thyroid cartilage plate, with a penetration depth of <2.0 mm in the thyroid cartilage plate (Figure 2), proper thyroid cartilage exposure is needed. The trend of EMG change is basically the same as that detected by the monitoring catheter surface electrodes (20) and is relatively unaffected by anesthesia, which is advantageous in procedures in which monitoring catheters cannot be used or in unplanned reoperations. However, it is important to note that these electrodes may be dislodged intraoperatively or may interfere with the surgical operation (21,22).
Recommendation 9. The typical recording electrodes used are monitoring catheter surface electrodes, and good contact between the surface electrodes and the bilateral vocal cords is required during their placement. (Grade of recommendation: A; level of evidence: C).
Recommendation 10. Thyroid cartilage needle electrodes can also be used as the recording electrodes. They can be placed in the lower-middle region of the anterior horn of the thyroid cartilage. (Grade of recommendation: B; level of evidence: C).
Other equipment and devices
Other equipment and devices of an IONM system include wire connection devices or interface boxes; anti-interference devices; and printers, keyboards, and storage devices. The anti-interference devices can shield the interference of unipolar electrosurgical equipment on the IONM system signals and prevent the formation of current loops that can lead to damage to the IONM system equipment.
Recommendation 11. Anti-interference devices should be connected to the unipolar electrosurgical equipment to shield against the interference of the unipolar electrosurgical equipment on the IONM system signals and prevent the system from short circuiting. (Grade of recommendation: A; level of evidence: C).
Establishment of an IONM system
Anesthesia management
Standardized anesthesia management is essential for ensuring the performance of monitoring, the steps for administering anesthesia could be performed by a nurse anesthetist or an anesthesiologist. Muscle relaxants can relax the laryngeal muscles and open the glottis and therefore are routinely used for tracheal intubation. However, improper use of muscle relaxants can weaken or destabilize EMG signals and thus affect the neuromonitoring performance. The type and dose of a muscle relaxant selected should meet the needs of tracheal intubation and simultaneously ensure that high-quality EMG signals can be obtained intraoperatively (1,13,19). During anesthesia induction, therefore, a short- to medium-acting nondepolarizing muscle relaxant should be selected, with a 1× 95% effective dose (ED95) typically being sufficient for routine surgeries, although the dosage can be appropriately increased for procedures with a longer duration and greater scope. During the maintenance phase of anesthesia, additional doses are generally not required, or the muscle relaxant can be added only in small amounts if necessary, and the principle of minimum dose should be followed (23,24). If the use of a muscle relaxant has a large impact on IONM, specific antagonists can be used to attenuate the myorelaxation. In addition, anticholinergic drugs can be used to suppress glandular secretion and prevent poor contact between recording electrodes and vocal cords due to excessive intraoperative secretions.
Recommendation 12. A short- to medium-acting nondepolarizing muscle relaxant should be selected. During the maintenance phase of anesthesia, a 1× ED95 is often given, and the dose may be increased appropriately. During the surgery, additional doses are generally not required, or the muscle relaxant can be added only in small amounts. If necessary, antagonists can be used to attenuate the myorelaxation so as to avoid any effects of the muscle relaxant on IONM. (Grade of recommendation: A; level of evidence: A).
Recommendation 13. For the purpose of anesthesia management, anticholinergic drugs can be used to suppress glandular secretion so as to maintain the good contact between the surface electrodes and vocal cords. (Grade of recommendation: B; level of evidence: C).
Placement of monitoring catheters
The appropriate type of monitoring catheters should be selected according to the patient’s gender, age, and body size. After induction of general anesthesia, the monitoring catheter should be placed under a video laryngoscope. The use of gel or oily lubricant to coat the catheter surface and the application of laryngeal spray for surface anesthesia should be avoided. The depth (21–22 cm for men and 20–21 cm for women) and angle of the catheter should be properly adjusted (25). Good contact between the surface electrodes and the vocal cords should be ensured. After placement, the catheter needs to be properly fixed. To prevent displacement of the recording electrodes following body position adjustment, the patient can also be placed in a surgical position before the monitoring catheter is indwelled.
Recommendation 14. Placing the monitoring catheter under video laryngoscope is advised. The use of catheter surface lubricants or laryngeal spray should be avoided. Attention should be paid to the angle and depth of the indwelling catheter, and good contact between the surface electrodes and the vocal cords should be ensured. (Grade of recommendation: A; level of evidence: C).
Connection of the monitoring circuit
The loop electrode at the stimulation end and the ground electrode at the recording end need to be placed in different areas (e.g., under the xiphoid process, the deltoid muscle, and the muscle groups of four extremities) according to typical procedures (3). Patch electrodes or subcutaneous needle electrodes can be selected, spaced about 1.0 cm apart, and properly fixed. The grounding electrode should be placed between the loop electrode and the operative area to avoid current overload and false-positive stimulation due to the intraoperative use of electrosurgical equipment.
Furthermore, the stimulation probe is placed in the sterile surgical area. The terminals of the stimulation probe and monitoring catheter are plugged into the appropriate interfaces on the connecting devices. After this, the anti-interference device can be connected.
Host parameter settings
The host can be turned on after all the devices are connected. First, the impedance of recording electrodes should be checked. Abnormal impedance values on the system indicates that the recording electrode has poor contact with the vocal cord or that the ground electrode is poorly connected, and appropriate adjustment is required (13). Subsequently, monitoring interface should be used to set the event threshold, stimulation current intensity, and other parameters as needed. Patient information and monitoring time can also be entered to facilitate information storage.
After the cutaneous flap is mobilized, the anterior cervical muscle tissue is probed using a stimulation current of 1.0 mA. It is important to ensure that the probe can effectively release current. After the surgical area is exposed, the carotid sheath area is probe with a stimulation current of 3.0 mA to obtain the EMG signal of the VN, which indicates the successful establishment of the monitoring system.
Recommendation 15. After the device connection is completed, the circuit connectivity should be tested to ensure that the monitoring system is successfully established. (Grade of recommendation: B; level of evidence: C).
Standard operating procedures of IONM
Pre- and postoperative laryngeal examination
Preoperative laryngeal examination (L1) is crucial to assessing and recording vocal fold function, which help guide the development of surgical strategies and provide a reference for postoperative changes in vocal fold function. At present, the vocal fold movement is mainly observed under a fiberoptic laryngoscope although ultrasound and other examination methods can also be used to assist in the assessment. Postoperative laryngeal examination (L2) can be applied optionally to compare the changes in vocal fold function, detect the occurrence of other complications, and formulate appropriate treatment plans (26).
Recommendation 16. Assessing vocal fold function using laryngoscopy or other examinations is recommended before surgery; after surgery, these examinations are optional. (Grade of recommendation: A; level of evidence: B).
Monitoring of laryngeal nerves
Monitoring of cervical VN
The cervical VN is the upstream nerve of the laryngeal nerve and needs to be routinely monitored intraoperatively. Obtaining a good VN signal is critical because it not only largely signals the successful establishment of the IONM system but also serves a baseline reference for subsequent changes in the laryngeal nerve signal. It can also be used to verify the integrity of the laryngeal nerve conduction function. In particular, when abnormal changes in the EMG signal occur intraoperatively, the VN signal can be used as a key decision-making input in the cause analysis (27). Therefore, bilateral VN monitoring is recommended.
Before the start of operation in the operative field, a 3.0-mA current is used to probe the EMG signal of the VN in the carotid sheath at the level of the inferior pole of the thyroid (defined as the V1 signal). If a VN signal cannot be obtained, the possibility of monitoring system-related problems, nerve degeneration, or nerve injury should be explored. If the amplitude of the VN signal is low, the sensitivity of nerve function assessment is weakened, and its causes should also be investigated. The surgical operation should not be performed before a higher amplitude is obtained. Upon the completion of the procedures in the operative field, a 3.0-mA current should be applied for remeasuring the EMG signal obtained at the VN (defined as the V2 signal).
When the VN is mapped during I-IONM, VN dissection or mobilization is usually not required. The EMG signal can be obtained by probing in the area between the common carotid artery and the internal jugular vein or in the adjacent areas. If the signal is not detected, VN dissection or a higher stimulation of current intensity may be performed or applied as appropriate. The VN inside the carotid sheath most often travels on the deep side between the arteries and veins (73%), although it may also travel on the deep side of the common carotid artery (15%), the deep side of the internal jugular vein (8%), and the superficial side between the arteries and veins (4%) (Figure 3) (28).

During C-IONM, dissection and mobilization of the VN is required for the placement of the VN continuous monitoring electrodes, which should be performed with care and caution. It is advisable to place the electrodes to the proximal position of the VN above the thyroid surgery area, which can reduce the influence of the electrodes on the thyroid surgery area.
Recommendation 17. During I-IONM, dissection of the carotid sheath is often not required for monitoring of the VN, and the EMG signal can be obtained merely by probing in the area between the common carotid artery and the internal jugular vein or in the adjacent areas. (Grade of recommendation: A; level of evidence: B).
Recommendation 18. Before the start of operation in the operative field, a 3.0-mA current should be used to probe the EMG signal of the VN in the carotid sheath at the level of the inferior pole of the thyroid (defined as the V1 signal). Upon the completion of the procedures at the operative field, a 3.0-mA current can be applied for remeasuring the EMG signal obtained at the VN (defined as the V2 signal). (Grade of recommendation: A; level of evidence: A).
Recommendation 19. During C-IONM, dissection and mobilization of VN is required, which must be performed with care and caution to avoid iatrogenic injury. (Grade of recommendation: B; level of evidence: C).
Monitoring of the RLN
The RLN originates from the thoracic segment of the VN. With a relatively high origination site, the right RLN loops under the right subclavian artery and moves back up through the neck. The left RLN arises at a lower position and loops around the aortic arch to the neck and then enters the larynx posterior to the cricothyroid articulation, innervating all laryngeal muscles except the cricothyroid muscle (CTM).
Most RLNs travel upward in the tracheoesophageal groove. Therefore, a 3.0-mA current can be applied to initially locate the RLN before anatomical exposure. A crossover method can be used; that is, the initial mapping can be performed below the lower pole of the thyroid gland perpendicular to the trachea to identify the site with the maximum signal strength. Subsequently, mapping along this site in a direction parallel to the direction of the trachea is performed; thus, the RLN can be roughly mapped through two crossover probing paths near the tracheoesophageal groove (Figure 4) (1). After the initial localization, a 1.0-mA current is applied for precise localization, and the obtained EMG signal is defined as the R1 signal. If the RLN is covered by thick tissues that interfere with the mapping, it can be appropriately exposed.
Up to 30% of RLNs have extralaryngeal branches (29), which can easily cause confusion and injury during surgery. A low-current monitoring method can be applied to distinguish the motor branches. That is, if the original stimulation current is not able to distinguish these branches, the current stimulation intensity can be lowered appropriately to reduce the current dispersion and improve the specificity of tiny nerve identification (for example, nerves with myoelectric signals are the motor branches) (30-32), thus enabling the more precise protection of the RLN trunk and its major branches.
During surgical operations in areas at high risk of RLN injury (e.g., the Berry ligament, areas invaded by tumors, and adhesions/scars), the RLN proximal point (Rp) monitoring method can be used, which involves the real-time stimulation of the RLN Rp during the dissection and the comparison of the Rp signal with the R1 signal (to determine whether the Rp signal is attenuated); this can provide the timely detection of any changes in amplitude and alert the surgeons to any risky operations (33,34). The RLN function can also be assessed indirectly by detecting the VN if the Rp is difficult to expose due to a large tumor or massive lymph node metastases in the central region. Upon the completion of the procedures at the operative field, a 1.0-mA current is applied for measuring the EMG signal (defined as the R2 signal) obtained at the nearest end of the exposed RLN.
Recommendation 20. A 3.0-mA current can be applied to initially locate the RLN below the lower pole of the thyroid gland by using a “crossover method” before anatomical exposure. (Grade of recommendation: A; level of evidence: B).
Recommendation 21. After the initial localization, a 1.0-mA current is applied for precise localization and appropriate exposure of the RLN, and the obtained EMG signal is defined as the R1 signal. Upon the completion of the procedures at the operative field, a 1.0-mA current is applied to measure the EMG signal (defined as the R2 signal) obtained at the nearest end of the exposed RLN. (Grade of recommendation: A; level of evidence: B).
Recommendation 22. For RLNs with extralaryngeal branches, a low-current monitoring method can be applied to distinguish the motor branches, thus enabling the more precise protection of the RLN trunk and its major branches. (Grade of recommendation: A; level of evidence: A).
Recommendation 23. During surgical operations in areas at high risk of RLN injury, the Rp monitoring method can be used to achieve the real-time monitoring at the nearest end of the exposed RLN. (Grade of recommendation: A; level of evidence: B).
Monitoring of the external branch of the EBSLN
The superior laryngeal nerve (SLN) arises from the cervical segment of the VN and is usually divided into an external and an internal branch at the level of the greater horn of the hyoid bone. The EBSLN is slender and highly variable. It usually travels with the superior thyroid artery, penetrating the inferior pharyngeal constrictor muscle or descending along its surface to innervate the CTM (30,31,35,36). The location of EBSLN is relatively fixed at the sternothyroid-larynx triangle, which has the sternothyroid as its lateral border, the inferior constrictor of the pharynx and CTM as the medial borders, and the superior pole of the thyroid gland as the lower border. Thus, it may be used as an anatomical landmark for the intraoperative localization of the EBLSN (Figure 5) (2,36).
Factors including high location of superior pole of the thyroid, oversized tumor mass, short and thick neck, large ratio of longitudinal thyroid diameter to neck length, heavy inflammatory adhesions, and reoperation can lead to an increased risk of EBSLN injury. Preoperative assessment can inform intraoperative identification and protection of the EBSLN (2). In some alternative anterior cervical approaches to the thyroid gland [e.g., the sternocleidomastoid intermuscular approach (37)], the use of IONM is more helpful in the visualization and protection of the EBSLN.
When the EBSLN is monitored with the surface electrodes of a monitoring catheter, its innervation areas can be mapped. Normally, while a CTM twitch is induced in all cases, EMG signals can be obtained in only a small proportion of patients, and these signals are characterized by low amplitude, short latency, and high waveform variability. Therefore, EBSLN monitoring should be performed with a CTM twitch as the primary indicator and an EMG signal as a secondary indicator (2,36).
Before the handling of vessels at the superior pole of the thyroid, a 1.0-mA current should be applied to the sternothyroid-larynx triangle for the initial localization of the EBSLN, which will induce a CTM twitch with or without an EMG signal (defined as the S1 signal). During dissection of the vessels at the superior pole of the thyroid, the innervation areas of the EBSLN should be mapped in a real-time manner. It is important to identify if the EMG signal is weakened (compared with the S1 signal). In cases whether the CTM twitch is absent, areas adjacent to the EBSLN should be promptly probed for any pulling, clamping, or misligation to avoid persistent injury. After ligation of the vessels at the superior pole of the thyroid, a 1.0-mA current should be applied in the innervation areas of the EBSLN to induce a CTM twitch, which can occur with or without an EMG signal (defined as the S2 signal).
Recommendation 24. The sternothyroid-larynx triangle may be used as an anatomical landmark for the intraoperative localization of the EBLSN. (Grade of recommendation: A; level of evidence: C).
Recommendation 25. Preoperative consideration of thyroid size, location of the superior pole, tumor size and location, and patient neck circumference/neck length can help assess the risk of EBSLN injury and inform intraoperative identification and protection of the EBSLN. (Grade of recommendation: A; level of evidence: B).
Recommendation 26. EBSLN monitoring and functional assessment should be performed with a CTM twitch as the primary indicator and EMG signal as the secondary indicator. (Grade of recommendation: A; level of evidence: A).
Recommendation 27. Before and after the handling of vessels at the superior pole of the thyroid, a 1.0-mA current should be applied to map the possible innervation areas of the EBSLN in the sternothyroid-larynx triangle, which will induce a CTM twitch with or without an EMG signal (defined as the S1 and S2 signals, respectively, before and after the handling). (Grade of recommendation: A; level of evidence: B).
Identification of NRLNs
In contrast to RLNs, NRLN is a condition in which the cervical VN reaches the larynx directly without looping around the great vessels. NRLN is a rare anatomical variant that occurs mostly on the right side (with an incidence of 0.6–0.9%) and may also be seen in the left side (about 0.04%). NRLN injury can easily occur if this variant is not identified and protected in timely fashion. Preoperative computed tomography can suggest the possibility of an NRLN, and the monitoring point comparison method and latency assessment method can be used to identify and protect the NRLN intraoperatively (38-40).
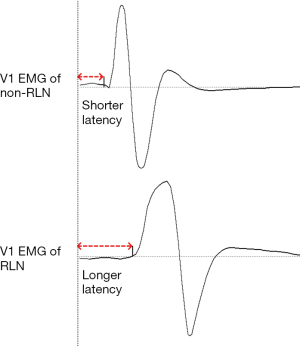
The monitoring point comparison method is used to predict the presence of an NRLN by comparing the EMG signals between the distal (inferior pole of the thyroid) and proximal (superior pole of the thyroid) monitoring points of the VN inside the carotid sheath (Figure 6). The latency assessment method relies on the varying length of nerve courses, resulting in different latencies of EMG signals. Since the course of the NRLN is dramatically shorter than that of normal RLN, the latency will be shorter (typically below 2.5 ms) for an NRLN after VN stimulation; in contrast, the latencies for a normal RLN are 5.0–7.0 and 3.1–4.7 ms, respectively, after the left and right VN are stimulated. Assessment of the latency duration can indicate the presence or absence of NRLNs (Figure 7) (39).
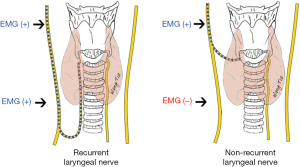
Recommendation 28. The monitoring point comparison method can be used to indicate the presence of an NRLN by comparing the EMG signals between the distal and proximal ends of the VN inside the carotid sheath. (Grade of recommendation: B; level of evidence: B).
Recommendation 29. The latency assessment method can identify the nerve types by assessing the length of latency. A VN latency below 2.5 ms may indicate the presence of an NRLN. (Grade of recommendation: B; level of evidence: B).
Standardized operating procedures of IONM
To ensure the effectiveness of the IONM technique and to better protect nerve function, standardized operating procedures of IONM should be followed, which include the preoperative and postoperative laryngeal examinations and the core “six-step” intraoperative procedure (Table 2) (1-3,19,36). The comparisons of R1 and R2 as well those of S1 and S2 with V1 and V2 signals can inform the changes in nerve function, assess the prognosis, and assist in the development of subsequent treatment plans.
Table 2
| Abbreviations | Specific procedure | Current intensity (mA) | Mapping site |
|---|---|---|---|
| L1 | Examine the larynx preoperatively to assess the basic condition of vocal fold function | – | – |
| V1 | Map the VN before the start of procedures at the operative field | 3.0 | Distal end of the nerve (at the level of the inferior pole of the thyroid) |
| R1 | Map the RLN after it is precisely located or initial exposed | 1.0 | Proximal end of the nerve (in the tracheoesophageal groove) |
| S1 | Map the EBSLN before the handling of vessels at the superior pole of the thyroid | 1.0 | The sternothyroid-larynx triangle |
| S2 | Map the EBSLN after the ligation of vessels at the superior pole of the thyroid | 1.0 | Innervation area |
| R2 | Map the RLN upon the completion of procedures at the operative field | 1.0 | Proximal end of the exposed nerve |
| V2 | Map the VN upon the completion of procedures at the operative field | 3.0 | Distal end of the nerve (at the level of the inferior pole of the thyroid) |
| L2 | Examine the larynx postoperatively, as appropriate | – | – |
IONM, intraoperative neuromonitoring; VN, vagus nerve; RLN, recurrent laryngeal nerve; EBSLN, external branch of the superior laryngeal nerve.
Recommendation 30. To ensure the effectiveness of the IONM technique and to better protect the nerve function, standardized operating procedures of IONM should be followed. (Grade of recommendation: A; level of evidence: A).
Monitoring of other cervical motor nerves
Other cervical motor nerves involved in thyroid and parathyroid surgeries include the accessory nerve, phrenic nerve, brachial plexus, hypoglossal nerve, and marginal mandibular branch of the facial nerve (41). During the monitoring of these nerves, stimulating electrodes are placed in the innervation areas to observe the contraction of the effector muscles, thus locating, identifying, and protecting these motor nerves (42,43).
Accessory nerve
The accessory nerve travels through neck levels II and V in the neck and is at risk of injury during cervical lymph node dissection. If the accessory nerve is difficult to expose, a 3.0- or 4.0-mA current can be applied to map it in its innervation areas, and the nerve can be located and identified if the contraction of the trapezius is observed. For procedures with a high risk of accessory nerve injury, adhesive electrodes or subcutaneous needle electrodes can also be placed on the trapezius to obtain the EMG signals of the trapezius, which can more accurately monitor changes in accessory nerve function (42-44).
Phrenic nerve
The phrenic nerve passes through neck level IV. The protection of the phrenic nerve is a priority during cervical lymphadenectomy, especially in reoperated cases or case in which the nerve is compressed by a huge mass or has severe deep cervical fascial adhesions. A 1.0- or 2.0-mA current can be applied in the anterior scalene muscle, and the nerve can be located and protected if the spasmodic contraction of the diaphragm (or hiccup) is observed. In addition, recording electrodes can also be placed at the xiphoid process or costal margin so that the changes in phrenic nerve function can be assessed with diaphragmatic EMG (42,43,45).
Brachial plexus
The brachial plexus is at risk of injury during thyroid surgery or cervical lymphadenectomy involving the supraclavicular fossa and during endoscopic- or robotic-assisted surgeries via axillary incisions. A 1.0- or 2.0-mA current can be applied to map the brachial plexus and its neighboring areas. The anatomic course and depth of the brachial plexus can be identified when the contraction of muscles in the upper arm and forearm is observed. In some other studies, somatosensory- and motor-evoked potential monitoring can be applied to monitor changes in brachial plexus function, which enables the real-time adjustment of upper limb position and thus the protection of the brachial plexus function (42,43,46).
Hypoglossal nerve
The hypoglossal nerve should be carefully protected during neck level II dissection. In particular, blind clamping in patients with lingual vein bleeding may injure the hypoglossal nerve. A 1.0- or 2.0-mA current can be applied to map the nerve at the deep posterior ventral surface of the digastric. The induced tongue tremor may help to locate and identify the hypoglossal nerve. Alternatively, in some studies, needle electrodes have been placed on both sides of the tongue to monitor the hypoglossal nerve function with an average current of 0.8 mA (42,43,47).
Marginal mandibular branch of the facial nerve
During cervical lymphadenectomy, injury to the marginal mandibular branch of the facial nerve may occur if the flaps are mobilized too highly or when a hook is used to assist in pulling the mandible and the posterior belly of the digastric upward. A 1.0-mA current can be applied to map the submandibular triangle, and marginal mandibular branch of the facial nerve may travel in this area if muscle contraction is observed around the mouth and the mandible (42,43,48).
Recommendation 31. For other cervical motor nerves, stimulating electrodes can be placed in the innervation areas of these nerves to observe the contraction of the effector muscles, thus locating, identifying, and protecting these motor nerves. (Grade of recommendation: A; level of evidence: B).
IONM in endoscopic- and robotic-assisted surgeries
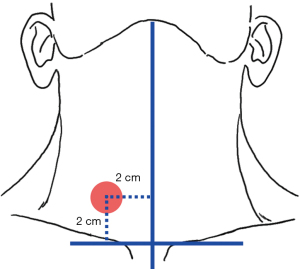
The surgical space is closed during endoscopic- and robotic-assisted surgeries, and therefore the use of stimulation probes is quite different from that in an open surgery. Thus, both “direct” and “indirect” IONM methods can be applied. The direct method, or the percutaneous puncture method, involves inserting the stimulation probe into the space of the thyroid surgery area for monitoring. In this method, the puncture site should be selected to open the anterior midline of the affected side of the neck 2.0 cm outward, the bilateral clavicle line should be translated up 2.0 cm to the center of the circle with a radius of 0.5 cm, the anterior cervical blood vessels should be avoided, and a 16-G needle should be used to poke the hole (Figure 8). The indirect method involves the application of different types of integrated stimulation electrodes for synchronized surgical operation and nerve mapping (3,49,50).
Analysis and management of abnormal EMG signals
Algorithm for assessment of abnormal EMG signals
EMG signal abnormality refers to the significant decrease in the amplitude of the intraoperative EMG signal provided that a satisfactory initial EMG signal has been obtained. LOS occurs when the amplitude decreases to below 100 µV (1,19,51).
If the initial EMG signal is normal but there is an abnormal intraoperative change, the ipsilateral VN should be stimulated to observe if there is any laryngeal muscle twitch. To accomplish this, a finger is placed on the posterior plate of the cricoid cartilage for palpation to determine if there is any contraction of the posterior cricoarytenoid. The presence of a laryngeal muscle twitch is confirmation that the stimulation end of the monitoring system is working properly, and the signal abnormality may thus originate from the recording end. If there is no laryngeal muscle twitch induced, the stimulation current intensity may not be sufficiently high. A 1.0- to 2.0-mA current can be applied to stimulate the sternocleidomastoid muscle; if there is contractile response, the contralateral VN should be further stimulated. If there is an EMG signal in the contralateral VN, ipsilateral nerve injury can be considered; if there is no EMG signal in the contralateral VN, a problem with the monitoring system is highly possible and needs to be addressed (7,13,27).
Recommendation 32. When an abnormal EMG signal occurs intraoperatively, prompt analysis to determine whether there is a monitoring system problem or nerve injury should be applied. (Grade of recommendation: A; level of evidence: A).
Common problems of the monitoring system and their management
If the signals are significantly weakened or cannot be measured when stimulating the proximal end of the exposed RLN and the contralateral VN, the monitoring system may have a problem, which should be analyzed and managed item by item according to the monitoring system troubleshooting algorithm (Figure 9).

Anesthesia-related problems
If there is a poor EMG signal at the start of the procedure or an abnormal EMG signal during the procedure, the anesthetist should be queried about the timing and dose of muscle relaxant administration and whether additional doses have been given intraoperatively. If there is no contractile response to CTM stimulation but the return current is normal, an overdose of muscle relaxant can be considered. In such cases, the surgical operation at the involved nerve region may be suspended or a specific muscle relaxant antagonist may be administered intravenously, and the procedure may be resumed after the muscle relaxation effect has diminished (13,52). In addition, difficult or repeated intubation may cause dislocation of the cricoarytenoid joint, resulting in abnormal changes in the EMG signal, although this happens rarely.
Recommendation 33. In case of overdose with muscle relaxant, the surgical operation at the involved nerve region may be suspended or a specific muscle relaxant antagonist may be administered, and the procedure may be resumed after the muscle relaxation effect has diminished. (Grade of recommendation: A; level of evidence: A).
Stimulation end-related problems
Stimulation end-related problems are conditions in which the stimulation current cannot be delivered to the nerve, resulting in failure to measure the EMG signal. The common causes have been previously noted and are outlined below (13,27,53).
Coverage of the operative field by fluids
Blood or other fluids on the nerve surface can cause the dispersion of stimulation current in all directions, and the actual current received by the nerve can thus decrease in strength. As a result, the current fails to reach the stimulation threshold to induce nerve depolarization, leading to a false negative stimulus, which can be confirmed by a contractile response to CTM stimulation and a normal return current. In such cases, fluids in the surgical field should be wiped off or the intensity of the stimulation current increased as appropriate.
Damaged or improperly connected stimulation probes
Damaged or loose insulation of the stimulation probe, loose connection between needle body and needle handle, and/or incorrect connection of the probe guidewire can cause the actual output of the stimulation current to be lower than the configured intensity, resulting in ineffective stimulation. In such cases, the probes should be checked or replaced.
Improperly configured or incorrectly connected stimulation channels in the system
Some neuromonitors have multiple stimulation channels, and the wire connection devices also have multiple stimulation channel interfaces. If a stimulation channel is incorrectly configured or connected, a closed loop cannot be formed, resulting in invalid stimulation. In such cases, the configuration of the host and the wire connections should be checked.
Blown fuse
If a stimulation probe is used to simultaneously excite unipolar electrosurgical devices, the high current generated can blow a fuse at the stimulation end, resulting in an interruption of the stimulation loop and invalid stimulation, as indicated by a lack of contractile response to CTM stimulation and a current output of 0. In such cases, the fuse in a connector or interface box should be checked and replaced.
Recommendation 34. Coverage of the nerve surface by blood or other fluids during monitoring should be avoided, as this may cause dispersion of the stimulation current and thus result in a poor signal. (Grade of recommendation: B; level of evidence: C).
Recommendation 35. Simultaneous excitation of unipolar electrosurgical devices should be avoided when using stimulation probes, as this may blow fuses and thus cause interruption of the stimulation circuit. (Grade of recommendation: B; level of evidence: C).
Recording end-related problems
The primary device on the recording end is the monitoring catheter. Close contact between the monitoring catheter surface electrodes and the bilateral vocal cords is a prerequisite for obtaining high-quality EMG signals. The common causes of problems at the recording end include (13,27,53,54).
Deflection of the monitoring catheter
A common cause of problems at the recording end is the deflection of the monitoring catheter, which occurs when one side of the recording electrode is detached from the vocal cord, resulting in a significant increase in electrode impedance on the corresponding side and a subsequent decrease in EMG signals. In such cases, the electrode-crossing method, in which one of the vocal fold electrodes on the left and right sides of the monitoring catheter is exchanged on the interface box, can be applied to increase the potential difference after a crossed electrode is formed and thus effectively improve the strength of the EMG signals (Figure 10). Therefore, when a problem at the recording end is suspected, the electrode-crossing method can be applied first to find the cause and improve the poor signals caused by the deflection of the monitoring catheter.
Improper depth of the monitoring catheter
When the monitoring catheter is too deep, the recording electrodes will be in direct contact with the laryngeal tissue below vocal cords and with the tracheal wall. Each recording electrode may reveal normal impedance; however, when the tracheal surface is stimulated, a nonnormal EMG waveform will be generated and the monitoring system will emit a unique tone, which can easily cause the operator to misjudge the neurological function. When the monitoring catheter is too shallow, the recording electrodes are suspended on both sides, and the impedance of the recording electrode will be too high or undetectable. When the nerve is stimulated, laryngeal muscle twitch will be visible, but the EMG signal cannot be detected or may be accompanied by obvious noisy signals.
If the monitoring catheter is deflected or at an inappropriate depth, the surgeon should work with the anesthetist to adjust the position of the monitoring catheter when stimulating the VN. The surgical operation can be resumed after the EMG signal is improved and the monitoring catheter is properly fixed.
Poor surface electrode contact
If a small monitoring catheter is used, poor contact between the surface electrodes and the vocal folds can lead to low-quality EMG signals. In addition, excessive secretions accumulating between the surface electrodes and the vocal cords can cause signal interference on the monitoring system. The use of gel or oil-based lubricants for coating the monitoring catheter may insulate the surface electrodes. Local anesthesia using a laryngeal spray can cause current dispersion. All of these conditions can undermine the monitoring results and require special attention in the preoperative placement of the monitoring catheter.
Recommendation 36. When a problem at the recording end is suspected, the electrode-crossing method can be applied first to find the cause and improve the poor signals caused by the deflection of the monitoring catheter. (Grade of recommendation: A; level of evidence: C).
Recommendation 37. If the monitoring catheter is considered to be deflected or at an inappropriate depth, the surgeon should work with the anesthetist to adjust the position of the monitoring catheter when mapping the VN. The surgical operation can be resumed after the EMG signal is improved. (Grade of recommendation: A; level of evidence: B).
Problems related to wire connections
Severe interfering waveforms appear on the monitor when a loop electrode or a ground electrode is accidentally dislodged during the surgery. Furthermore, severe interfering waveforms may also occur if the host is not connected to a well-grounded 3-phase alternating current (AC) power socket. In such cases, repositioning the electrode(s) or replacing the power socket can solve these problems.
Recommendation 38. For severe interfering waveforms encountered intraoperatively, the loop electrodes and ground electrodes should be first checked to see if they are dislodged. (Grade of recommendation: B; level of evidence: C).
Recommendation 39. The host should be connected to a well-grounded 3-phase AC power socket to avoid interference from the electric circuit. (Grade of recommendation: B; level of evidence: C).
Causes of nerve injury and their intraoperative management
Causes of RLN injury
The possibility of RLN injury should be considered after the monitoring system-related problems are ruled out item by item. If an EMG signal is detected after stimulation of the RLN entry point into the larynx but no EMG signal is detected after stimulation of the ipsilateral VN, type I RLN injury (i.e., segmental or localized RLN injury) is highly possible, and the injury site can be located along the nerve course from the entry point into the larynx to the nearest end. Type I injuries are most commonly caused by overstretching, pressure, clamping, or thermal injuries of the nerve, with overstretching being the leading cause (55,56). If there is no EMG signal detected throughout the stimulation of the RLN and ipsilateral VN but the EMG signal is detected after the stimulation of the contralateral VN, type II injury (or a global injury) is indicated. Type II injuries have no clear point of injury or well-defined pathogenic mechanism; however, they may be caused by injury to the intralaryngeal RLN branches (13,27).
Recommendation 40. When an RLN injury is suspected, the RLN course from the entry point into the larynx to the nearest end and the bilateral VN can be mapped for any abnormal signals so as to identify the type and cause of the injury. (Grade of recommendation: A; level of evidence: A).
Intraoperative management of RLN injury
Injuries to the RLN are most commonly associated with nerve overstretching, during which the EMG signal often gradually decreases in a manner correlating with the degree of stretching. The EMG signal may return normal if the overstretching is detected and managed in timely fashion. Therefore, 50% of the R1 signal amplitude is set as the adverse event threshold for RLN injury, and a drop of intraoperative EMG signal amplitude below the 50% threshold can serve as an early alert for the occurrence of nerve injury (6,13,15,27).
In such cases, the surgical operation should be suspended. A period of 20 to 30 minutes should be reserved to observe signal recovery and analyze the possible causes. After the EMG signal is fully or partially recovered, the procedure can be continued, and improper maneuvers should be corrected to avoid superimposed injuries. A persisting LOS often suggests a high risk of postoperative VCP. In this case, adjustments to the surgical plan or the scheduling of a staged surgery should be considered according to the diagnosis and treatment needs (7,57,58).
Recommendation 41. A drop to 50% of the R1 signal amplitude should be set as the adverse event threshold for RLN injury, which can serve as an early alert for the occurrence of RLN injury. (Grade of recommendation: A; level of evidence: B).
Recommendation 42. When there is a drop of intraoperative EMG signal amplitude below the 50% threshold, the surgical operation should be suspended, and the possible cause should be analyzed and treated accordingly. (Grade of recommendation: A; level of evidence: B).
Conclusions
Laryngeal/cervical nerve protection in thyroid related surgery is systematic, involving a variety of comprehensive management, and has a great dependence on the surgical skills and clinical experience of the surgeon. Preoperative comprehensive risk assessment, meticulous operation of capsule anatomy, reasonable application of advanced energy instruments, and improvement of protection and prevention awareness are the preconditions for effective prevention of nerve injury. As an auxiliary tool, IONM technology plays a positive role in the protection of intraoperative nerve function. The standardized application of IONM technology is the key to ensure the benefits. In practice, it is necessary to consolidate the theoretical knowledge and skills in basic principles, parameter interpretation, system establishment, standard operation, cause analysis and treatment of abnormal electromyography signals, and rationally apply it according to the actual operation situation.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the RIGHT reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-284/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-284/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-284/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chinese Thyroid Association, College of Surgeons, Chinese Medical Doctor Association. Clinical guidelines on intraoperative neuromonitoring in thyroid and parathyroid surgeries (Chinese edition). Chinese Journal of Practical Surgery 2013;33:470-4.
- Chinese Thyroid Association, College of Surgeons, Chinese Medical Doctor Association. Chinese Research Hospital Association Thyroid Disease Committee; Committee of Thyroid Surgical Equipment, Branch of Surgical Equipment, China Association of Medical Equipment. Expert consensus on protection and monitoring of the external branch of the superior laryngeal nerve during thyroid and parathyroid surgeries (2017 version). Chinese Journal of Practical Surgery 2017;37:1243-9.
- Chinese Thyroid Association, College of Surgeons, Chinese Medical Doctor Association; Chinese Research Hospital Association Thyroid Disease Committee; Branch of Clinical Practical Technology, China International Exchange and Promotive Association for Medical and Health Care, et al. Expert consensus on neurophysiological monitoring in robotic thyroid and parathyroid surgeries (2019 version). Chinese Journal of Practical Surgery 2019;39:1248-53.
- Piggott T, Morgan RL, Cuello-Garcia CA, et al. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) notes: extremely serious, GRADE's terminology for rating down by three levels. J Clin Epidemiol 2020;120:116-20. [Crossref] [PubMed]
- Zhao YS, Zhao ZH, Sun H. Evolution, innovation, and standards of intraoperative nerve monitoring technology in thyroid surgery. Journal of Clinical Surgery 2020;28:217-20.
- Liu XL, Li CL, Zhao YS, et al. Functional recovery after recurrent laryngeal nerve injury on different electromyography thresholds during thyroid surgery. Chinese Journal of Surgery 2017;55:853-6. [PubMed]
- Zhao YS, Kou JD, Liang N, et al. Study on the best surgical strategy after unilateral nerve EMG signal loss in bilateral thyroid surgery. Chinese Journal of Practical Surgery 2022;42:695-9.
- Sun H, Liu XL. Etiology and emergency management of asphyxia after thyroidectomy. Chinese Journal of Operative Procedures of General Surgery 2013;7:254-7. (Electronic Edition).
- Sun H, Liu XL, Fu YT, et al. Application of intraoperative neuromonitoring during complex thyroid operation. Chinese Journal of Practical Surgery 2010;30:66-8.
- Liu XL, Sun H, Zheng ZL, et al. Application and current status of intraoperative recurrent laryngeal nerve monitoring during thyroid surgery. Chinese Journal of General Surgery 2009;18:1187-90.
- Sun H, Tian W, Jiang K, et al. Clinical guidelines on intraoperative neuromonitoring during thyroid and parathyroid surgery. Ann Transl Med 2015;3:213. [PubMed]
- Gavilán J, Gavilán C. Recurrent laryngeal nerve. Identification during thyroid and parathyroid surgery. Arch Otolaryngol Head Neck Surg 1986;112:1286-8. [Crossref] [PubMed]
- Sun H, Diongi G. Intraoperative Neuromonitoring in Thyroid Surgery. Changchun: Jilin Science and Technology Press; 2017:28-9, 61-82, 86-94.
- Zhao Y, Zhao Z, Wang T, et al. The area under the waveform of electromyography for monitoring the external branches of the superior laryngeal nerve during thyroid surgery. Gland Surg 2021;10:143-53. [Crossref] [PubMed]
- Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery 2014;155:329-39. [Crossref] [PubMed]
- Wu CW, Liu X, Barczyński M, et al. Optimal stimulation during monitored thyroid surgery: EMG response characteristics in a porcine model. Laryngoscope 2017;127:998-1005. [Crossref] [PubMed]
- Zhao QZ, Wang CX, Wang Y, et al. Clinical application of special instruments for endoscopic thyroid surgery. Chinese Journal of Practical Surgery 2018;38:690-3.
- Wang D, He QQ, Zhu J, et al. Improvement of the use of intraoperative recurrent laryngeal nerve monitoring in Da Vinci robot thyroid surgery. International Journal of Surgery 2018;45:836-8, 866.
- Randolph GW, Dralle HInternational Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Zhao Y, Li C, Zhang D, et al. Experimental study of needle recording electrodes placed on the thyroid cartilage for neuromonitoring during thyroid surgery. Br J Surg 2019;106:245-54. [Crossref] [PubMed]
- Zhao Y, Zhang D, Zhou L, et al. Proprieties of adhesive surface arrays to thyroid cartilage for recurrent laryngeal nerve monitoring. Ann Transl Med 2021;9:690. [Crossref] [PubMed]
- Zhao YS, Li SJ, Zhao ZH, et al. Application of thyroid cartilage single needle recording electrode in intraoperative nerve monitoring of thyroid surgery. Chinese Journal of Experimental Surgery 2020;37:2302-5.
- Chen P, Liang F, Li LY, et al. The effect of different concentrations of rocuronium bromide induction on the introperative recurrent laryngeal nerve monitoring of the patients with thyroid operation. Chinese Journal of Laboratory Diagnosis 2014;18:1460-2.
- Liu X, Sun ZR, Yang SW, et al. Application of Shugeng glucose sodium in neuromonitoring of radical thyroid cancer surgery. Chinese Journal of Cancer 2019;29:212-7.
- Nakanishi T, Yoshimura M, Sakamoto S, et al. Postoperative laryngeal morbidity and intubating conditions using the McGRATH™ MAC videolaryngoscope with or without neuromuscular blockade: a randomised, double-blind, non-inferiority trial. Anaesthesia 2018;73:990-6. [Crossref] [PubMed]
- Chandrasekhar SS, Randolph GW, Seidman MD, et al. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg 2013;148:S1-37. [Crossref] [PubMed]
- Randolph GW, Kamani D, Wu CW, et al. Surgical Anatomy and Monitoring of the Recurrent Laryngeal Nerve. Surgery of the Thyroid and Parathyroid Glands (Third Edition). Amsterdam: Elsevier; 2021:326-59.e10.
- Dionigi G, Chiang FY, Rausei S, et al. Surgical anatomy and neurophysiology of the vagus nerve (VN) for standardised intraoperative neuromonitoring (IONM) of the inferior laryngeal nerve (ILN) during thyroidectomy. Langenbecks Arch Surg 2010;395:893-9. [Crossref] [PubMed]
- Yin C, Song B, Wang X. Anatomical Variations in Recurrent Laryngeal Nerves in Thyroid Surgery. Ear Nose Throat J 2021;100:930S-6S. [Crossref] [PubMed]
- Wang K, Cai H, Kong D, et al. The Identification, Preservation and Classification of the External Branch of the Superior Laryngeal Nerve in Thyroidectomy. World J Surg 2017;41:2521-9. [Crossref] [PubMed]
- Zhao Y, Zhao Z, Zhang D, et al. Improving classification of the external branch of the superior laryngeal nerve with neural monitoring: a research appraisal and narrative review. Gland Surg 2021;10:2847-60. [Crossref] [PubMed]
- Zhao Y, Li C, Liu X, et al. Investigation on EMG Profiles of the Superior Laryngeal Nerve in a In Vivo Porcine Model. J Invest Surg 2020;33:596-604. [Crossref] [PubMed]
- Yuan Q, Wu G, Hou J, et al. Correlation Between Electrophysiological Changes and Outcomes of Vocal Cord Function in 1764 Recurrent Laryngeal Nerves with Visual Integrity During Thyroidectomy. Thyroid 2020;30:739-45. [Crossref] [PubMed]
- Zhao YS, Liu XL, Wang T, et al. The function of recurrent laryngeal nerve and movement of vocal cords in thyroid surgery. Chinese Journal of Bases and Clinics in General Surgery 2015;22:784-7.
- Lu KN, Ding JW, Zhang Y, et al. The Anatomical and Clinical Significance of the Superior Laryngeal Nerve. Otolaryngol Head Neck Surg 2021;165:690-5. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123:S1-14. [Crossref] [PubMed]
- Fu J, Zhao Y, Sun H, et al. The feasibility of laryngeal nerve protection during thyroidectomy using sternocleidomastoid intermuscular approach with intraoperative neuromonitoring: a case series and step-by-step description of surgical procedure. Gland Surg 2022;11:1665-72. [Crossref] [PubMed]
- Henry BM, Sanna S, Graves MJ, et al. The Non-Recurrent Laryngeal Nerve: a meta-analysis and clinical considerations. PeerJ 2017;5:e3012. [Crossref] [PubMed]
- Wang T, Dionigi G, Zhang D, et al. Diagnosis, anatomy, and electromyography profiles of 73 nonrecurrent laryngeal nerves. Head Neck 2018;40:2657-63. [Crossref] [PubMed]
- Wang Y, Ji Q, Li D, et al. Preoperative CT diagnosis of right nonrecurrent inferior laryngeal nerve. Head Neck 2011;33:232-8. [Crossref] [PubMed]
- Xu ZG, Liu SY. Expert consensus on cervical lymph node dissection in differentiated thyroid cancer (2017 version). Chinese Journal of Practical Surgery 2017;37:985-91.
- Li F, Zhou L, Liu XL, et al. Application and evaluation of nerve monitoring technology in thyroid and neck surgery. Chinese Journal of Practical Surgery 2015;35:901-3.
- Li F, Sun H. The application of intraoperative neuromonitoring in lateral neck dissections for thyroid cancers. Ann Thyroid 2019;4:15-22. [Crossref]
- Lanisnik B, Zargi M, Rodi Z. Electrophysiologic analysis of injury to cranial nerve XI during neck dissection. Head Neck 2016;38:E372-6. [Crossref] [PubMed]
- Duque CS, Dueñas JP, Marulanda M, et al. Phrenic nerve stimulation during neck dissection for advanced thyroid cancer involving level IV: is it worth doing it? Updates Surg 2017;69:83-7. [Crossref] [PubMed]
- Huang S, Garstka ME, Murcy MA, et al. Somatosensory evoked potential: Preventing brachial plexus injury in transaxillary robotic surgery. Laryngoscope 2019;129:2663-8. [Crossref] [PubMed]
- Duque CS, Londoño AF, Penagos AM, et al. Hypoglossal nerve monitoring, a potential application of intraoperative nerve monitoring in head and neck surgery. World J Surg Oncol 2013;11:225. [Crossref] [PubMed]
- Chiang FY, Lien CF, Wang CC, et al. Proposals for Standardization of Intraoperative Facial Nerve Monitoring during Parotid Surgery. Diagnostics (Basel) 2022;12:2387. [Crossref] [PubMed]
- Zhang D, Li F, Wu CW, et al. Percutaneous probe stimulation for intraoperative neuromonitoring in total endoscopic thyroidectomy: A preliminary experience. Head Neck 2017;39:1001-7. [Crossref] [PubMed]
- Zhang D, Wang C, Wang T, et al. Clinical Experience of Use of Percutaneous Continuous Nervemonitoring in Robotic Bilateral Axillo-Breast Thyroid Surgery. Front Endocrinol (Lausanne) 2021;12:817026. [Crossref] [PubMed]
- Calò PG, Medas F, Conzo G, et al. Intraoperative neuromonitoring in thyroid surgery: Is the two-staged thyroidectomy justified? Int J Surg 2017;41:S13-20. [Crossref] [PubMed]
- Lu IC, Tan H, Wu SH, et al. A comparison between cisatracurium and rocuronium-induced neuromuscular block on laryngeal electromyography recovery after neostigmine reversal in a porcine model. Front Endocrinol (Lausanne) 2022;13:875597. [Crossref] [PubMed]
- Zang Y, Tian W, Yao J, et al. Analysis of signal loss of intraoperative neuromonitoring in thyroid surgery. Chinese Journal of Practical Surgery 2017;37:1173-5.
- Kim HY, Tufano RP, Randolph G, et al. Impact of positional changes in neural monitoring endotracheal tube on amplitude and latency of electromyographic response in monitored thyroid surgery: Results from the Porcine Experiment. Head Neck 2016;38:E1004-8. [Crossref] [PubMed]
- Chiang FY, Lee KW, Chen HC, et al. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 2010;34:223-9. [Crossref] [PubMed]
- Zhao Y, Li C, Wang T, et al. Translational Study to Standardize the Safe Use of Bipolar Forceps, LigaSure, Sonicision and PlasmaBlade Around the Recurrent Laryngeal Nerve in Thyroid Surgery. Surg Technol Int 2018;31:sti32/990.
- Schneider R, Randolph GW, Dionigi G, et al. International neural monitoring study group guideline 2018 part I: Staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope 2018;128:S1-S17. [Crossref] [PubMed]
- Kou JD, Zhao YS, Liang N, et al. The influence of loss of intraoperative nerve monitoring signal on decision-making of total thyroidectomy: similarities and differences between Chinese and western perspectives. Chinese Journal of Endocrine Surgery 2022;16:503-5.







