
Optimizing aesthetic results in autologous breast reconstruction
Introduction
“As to the closure of the wound I should not care to say ‘Beware of the man with the plastic operation’.”—Halsted (1).
In 1907, when breast cancer was typically diagnosed at an advanced stage and surgery was the sole treatment, Halsted proposed the radical mastectomy and warned his colleagues against closing the breast wound “by any plastic method”. Halsted’s concern was understandable considering at that time local recurrence rates were greater than 50 percent and most surgeons had never seen a patient cured of breast cancer (1). Fortunately, we have seen incredible advances in our approach to breast cancer over the past century. The advent of radiation and hormonal therapies, breast cancer screening, typing of tumor receptors and genetic testing, now provide most patients with the hope for cure or avoidance of cancer altogether. Such advances have made quality of life much more important after treatment and have led to equally incredible advances in breast reconstruction, to the point where reconstructive goals have altered the way mastectomies are now performed. Over the last decade, the rate of breast reconstruction has increased by 75% with greater than 130,000 reconstructions performed in the United States in 2020 alone.
With increased survival after breast cancer, the importance of the aesthetic outcome and quality of life after treatment has similarly increased. Mastectomy leads to an undeniable disruption of a patient’s connection with their femininity, as well as severe psychosocial distress, and a dramatic effect on sexual well-being (2,3). A shift towards patient-centered care has motivated plastic surgeons to adapt their approaches to reconstruction integrating aesthetic principles to the process of recreating a breast mound in order to provide patients with a long-term, natural, and optimal result.
Microsurgery unlocked the potential to move tissue from remote sites on the body to the breast, while minimizing morbidity. However, as experience and expertise in microsurgery has grown, the surgical feat of successfully transferring tissue to restore breast volume is no longer considered an adequate endpoint for aesthetic breast reconstruction. Microsurgical tissue transfer should provide patients with a permanent, natural feeling and natural appearing breast reconstruction that may even exceed the aesthetics of a patient’s natural breasts. These aesthetic goals should also extend to the donor site, where adequate contour improvement is sought to offset the price of the donor site scar and the morbidity is minimized.
In this article we will explore some of the approaches and considerations in achieving optimal aesthetics in autologous reconstruction. We will focus on the deep inferior epigastric perforator (DIEP) flap as this is the most common technique in use, however these principles may also be applied to other flaps.
Preoperative assessment
Aesthetic breast reconstruction begins with a thorough preoperative assessment, history, and physical exam. It is important to identify existing asymmetries between breasts including volume, nipple position, and inframammary fold height. The underlying chest contour and skeletal structure can also distort breast symmetry and should be examined carefully and taken into consideration. Deformities such as pectus excavatum or carinatum can create severe breast asymmetry (4). Prior surgeries or radiation may impact blood supply and affect the viability of breast skin as well as the incision planning. Donor site contour, scars, tissue laxity and tissue thickness are also assessed to determine adequacy for breast restoration.
Breast footprint
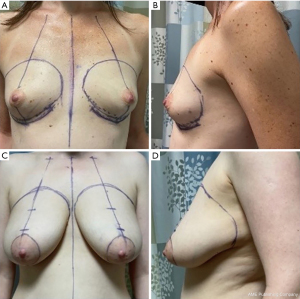
Blondeel et al. discussed the importance of identifying the footprint of the breast on the chest wall in achieving an aesthetic reconstruction (5). There is significant variability in the size of the breast footprint depending on a patient’s body habitus, chest diameter, and height. However, there are common anatomic landmarks that represent the borders of the footprint. The breast footprint typically starts near the sternal border and gently curves upward toward the upper chest several centimeters below the clavicle. The upper limit can be visualized by gently pushing upward on the breast to see where the fullness ends. This transitions laterally across the lateral border of the pectoralis major muscle. The lateral extent of the breast may be harder to discern in obese individuals due to a fat roll that can exist here, but often a zone of adherence is present that can be seen as a slight indentation where the breast ends. This is sometimes more evident when the patient lies supine, and the breast falls laterally. When marking the lateral border, it is important to note that the lateral border should not extend beyond the mid-axillary line. Even if tissue is removed in this region, if the reconstruction extends too far laterally the patient will complain of fullness against their arm. Inferiorly, the footprint is delineated by the inframammary fold, which can be followed medially to a few centimeters from the sternal midline. Marking the borders of the breast and avoiding resection of tissue beyond the borders is important in achieving an optimal aesthetic outcome and ideally should be reviewed with the breast surgeon preoperatively (Figure 1).
The breast conus: approaches to restoring core projection of the breast
Critical to an aesthetic breast form is establishing adequate central prominence of the breast, what can be referred to as the “core projection” of the breast. Though implant-based reconstruction remains the most common method in the United States, patients report higher satisfaction following autologous reconstruction, owing to the softer and more natural feeling breasts it can provide (6). However, flap tissue may not have the inherent core projection that an implant has, so this must be addressed when considering the reconstruction. After reviewing with the patient their desired breast volume, the surgeon must assess the donor site to ensure enough tissue is available to achieve these goals. Volume must be considered in the context of the base dimensions or “footprint” of the breast as well as its adequacy to fill the skin envelope and attain a desirable nipple position.
There are several techniques that can be employed to enhance core projection of the flap itself depending on whether the reconstruction is unilateral or bilateral and whether it is immediate or delayed. One approach is to simply tuck the corners of the flap inward at the inframammary fold to round the bottom of the flap and add some projection. Another entails a purse string type suture underneath the breast or around the periphery of the flap to cinch the circumference and thus increase projection (7,8). Certain flaps, such as the transverse upper gracilis (TUG) flap may also be designed in a manner that will allow for folding upon itself. These techniques require adequate flap width, in addition to flap volume, in order to maintain the breast footprint while achieving core projection.
For patients with inadequate donor site tissue to achieve an aesthetic result from a single flap, stacked flaps may be considered. For patients undergoing unilateral breast reconstruction, stacked DIEP flaps are often used, however stacked thigh flaps are another popular alternative. In the case of stacked DIEP flaps, this may be configured as two separate flaps or as a single, dual-pedicled flap that is folded to provide increased projection (Figure 2). In cases of bilateral reconstruction, a four-flap technique can be used (9). In this method, DIEP flaps can be stacked with profunda artery perforator (PAP) flaps, TUG flaps or lumbar artery perforator (LAP) flaps on each side. Patient reported outcomes have revealed that overall levels of satisfaction in four-flap reconstruction are similar to bilateral DIEP reconstruction, despite the additional donor site. Additionally, for patients undergoing DIEP plus PAP reconstruction, a majority of patients report an aesthetic improvement in the contour of their thighs (10).
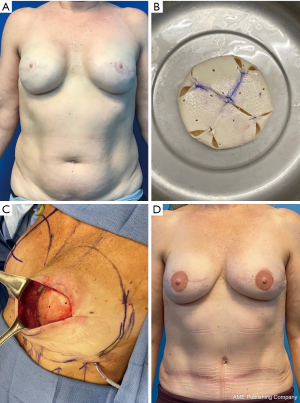
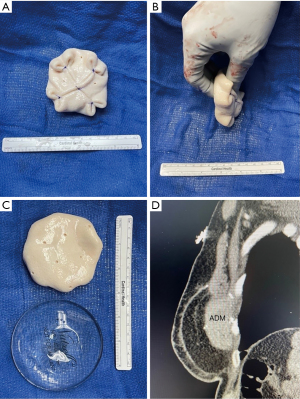
If this is not possible with the available donor sites, then additional maneuvers must be considered. The introduction of an implant to autologous reconstruction, termed hybrid reconstruction, can provide additional volume when the flap is inadequate. The traditional hybrid approach is the combination of the latissimus dorsi flap with an implant, however patients are burdened with a less aesthetic scar location and the potential for animation deformity. Additionally, the majority of the volume is provided by the implant in this hybrid approach, with the latissimus providing coverage that often atrophies and contracts with time. In recent years, there is growing practice of combining DIEP flaps, instead of the latissimus flap, with an implant. This allows for better soft tissue coverage, avoidance of muscle sacrifice and a more easily concealed scar. Additionally, in the DIEP flap and implant approach, the majority of the volume is provided by the flap, while the implant serves to increase the core projection and improve the shape of the breast (Figure 3). With a higher volume of fat superficial to the implant, the risk of atrophy and rippling is significantly lower when compared to the latissimus flap. Studies have shown that this approach is safe and does not increase the risk of mastectomy skin flap necrosis (11). The use of acellular dermal matrix (ADM), to support the implant in position under a flap has been adopted from its use in prepectoral breast reconstruction (12). More recently, our group has described the Hybrid Prepectoral Acellular Dermis (HyPAD®) technique and presented our experience using thicker pieces of ADM alone, to add core projection to the flap, thus avoiding the need for an implant (Figure 4) (13). This method has allowed for volume stability overtime, while also protecting the vascular pedicle should the patient desire further augmentation at a later stage of reconstruction.
Skin envelope and nipple-areolar complex (NAC) position
Management of the skin envelope has changed greatly over time due to improved screening and genetic testing to identify patients at early-stage disease or even before they develop cancer; improved adjuvant treatments that have decreased our reliance on aggressive surgery, improved survival that has increased the emphasis on quality of life after treatment and advances in reconstructive techniques that have raised the overall standard for aesthetic outcomes after surgery. One of the first changes was increasing the preservation of the skin envelope, which was followed by preservation of the NAC (14). With preservation of the NAC, attention turned to scar location with the inframammary approach gaining in popularity, due to its lower associated risk when compared to Wise pattern or periareolar technique (15,16). More recently, minimally invasive approaches using endoscopic and robotic techniques have developed to further reduce and hide the scar.
The patient’s skin envelope plays a critical role in the final shape of the breast. As evidenced by the natural progression of aging skin, the quality of the breast envelope plays a key role in maintaining the breast volume in the appropriate position. Of course, a history or plan for future radiation can affect the quality of the skin envelope leading to a tighter pocket. While irradiated skin is certainly at higher risk of wound-healing complications, oftentimes radiation has an unexpected effect of tightening the envelope and creating a higher and lifted breast mound, resembling a more youthful shape that some patients prefer.
Proper position of the skin envelope and NAC may be facilitated sometimes by simply using adhesive dressings to reposition the breast skin and nipple position on the underlying flap. Excess inferior skin may be de-epithelialized and imbricated or excised depending on the adequacy of blood supply to the remaining breast skin. More significant cases of volume skin mismatch or NAC malposition may need to be addressed in a staged fashion (discussed below). Preliminary breast reduction or mastopexy may be performed, if oncologic considerations allow, and can facilitate matching the skin envelope and NAC position with the volume available or desired for the reconstruction. Previously, free NAC grafting was used in patients that presented as oncologic candidates for nipple sparing mastectomy (NSM), however had a great degree of ptosis leading to an anatomic limitation (17,18). The staged breast reduction/mastopexy approach has gained significant popularity over free NAC grafting, avoiding the difficulty of determining NAC positioning at time of primary reconstruction.
Donor site selection
Since Hartrampf first popularized the rotational transverse rectus abdominis myocutaneous flap (TRAM), the use of the lower abdominal tissue for autologous breast reconstruction has been the gold standard (19). Allen went on to describe the DIEP flap in 1994, which quickly became the clear first choice in microvascular breast reconstruction (20). In regard to aesthetics, many women have an excess of skin and fat in the infraumbilical region and are enthusiastic about the prospect of contouring their abdomen. Subsequent advances in microsurgical technique led to a more thoughtful approach to donor site selection, with an emphasis on minimizing morbidity, reliable vasculature and of course, reproducibility.
For patients with a history of abdominal surgery or those with insufficient abdominal tissue to recreate an aesthetically appropriate breast mound, the gluteal and thigh regions have emerged as the most popular second choice. Much like the abdominal donor site, the progression of flaps in these areas, namely the superior gluteal artery perforator (SGAP) flap, inferior gluteal artery perforator (IGAP) flap, then TUG flap, and most recently the PAP flap, illustrate the evolution in donor site location and design (21). The gluteal flaps (SGAP, IGAP) may have the unwanted effect of creating a concavity in the buttock. More recently, the LAP flap has allowed surgeons to move the flap location above the buttock to a region where the resulting scar may be more visible but the concavity in the donor site is more aesthetically acceptable. Flaps harvested from the inner thigh (TUG, PAP) can have the welcome effect of contouring the inner thigh, however one must avoid labial spreading by marking the upper border of the flap 1–2 cm inferior to the groin crease and ensuring a tension free closure by tacking down to Colles fascia. If more tissue is required than can be obtained with the traditional transverse flap design, a fleur-de-lis flap design may allow additional volume to be taken without increasing the tension on the transverse closure. This technique adds additional central flap volume by taking a central vertical limb of tissue with the flap. The scar is well concealed, however, the T-junction it creates can be prone to minor dehiscence (22). The added central volume also allows for improved breast shaping and reduces the need to extend the transverse scar too far anteriorly or posteriorly (22). The traditional TUG and PAP flaps can also be coned to provide improved shape to the breast mound as long as the base width of the breast is not too wide (23).
Staging and revision procedures
The purpose of the principal operation is to achieve a shape as close to a normal breast as possible, establishing the borders of the breast footprint and recreating the core projection. However, the best aesthetic outcome cannot be achieved in a single stage. These secondary procedures typically occur at least 3 months postoperatively when the flap edema and inflammation have subsided and scars have matured, and the reconstructive effort can be judged as a whole, taking into account both the breasts and the donor site. The potential adjunct procedures that are often considered include nipple reconstruction, scar revisions, volume adjustments, liposuction, fat grafting or shape corrections.
The NAC serves as the central landmark of the breast. NAC reconstruction is an integral part of the breast reconstruction process, as its restoration has a critical psychological impact on the patient and symbolizes the culmination of their reconstructive journey. The goal of reconstruction is symmetry in position, size, shape, texture, color, and projection (24,25). Various methods of nipple reconstruction have been described with local flaps and areolar tattooing being the most popular to date. The most challenging aspect of nipple reconstruction is loss of projection over time. To account for this, overcorrection by 25–50% is advisable. If nipple augmentation is necessary, autologous fat grafting has been used (26).
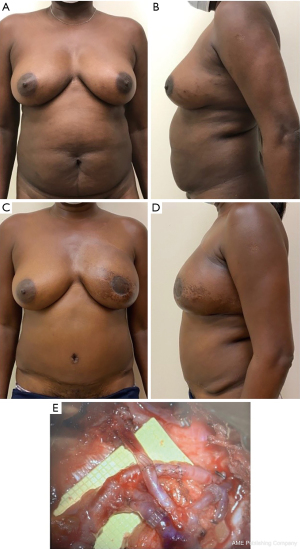
One of the major shifts in breast reconstruction has been preservation of the NAC at the time of mastectomy. For the appropriately selected patient, NSM is oncologically safe and can provide an exceptional aesthetic result. The indications for NSM have expanded over the years, with inflammatory breast cancer and malignancy involving the nipple being the only absolute contraindications, making it safe for both breast cancer patients and those with genetic mutations seeking prophylactic mastectomy (27). While this has provided plastic surgeons with the potential to achieve the most natural appearing reconstructive outcomes, it also may present certain challenges, in particular, when the existing NAC location is not in the ideal location for the reconstruction. In that case, surgeons have two options to consider. If the patient is undergoing prophylactic mastectomies or their tumor is amenable to an oncoplastic resection, a mastopexy or breast reduction may be performed initially, followed by mastectomy as a second procedure 10–12 weeks later. Some surgeons have performed the mastectomy at shorter intervals of 3 to 4 weeks, however, we have had a few instances of nipple loss with that approach so opt for a longer interval (Figure 5) (28,29).

The other options are to adjust the NAC position as best as possible at the time of nipple-sparing mastectomy and autologous reconstruction, and then come back 10–12 weeks later to revise the skin envelope and NAC position secondarily. Compared to implant reconstruction, autologous breast reconstruction has the advantage of revascularizing the overlying breast skin and NAC. This allows the plastic surgeon to comeback 10–12 weeks later to circumscribe and reposition the NAC and excise excess breast skin in a mastopexy type fashion, as long as the NAC maintains a blood supply from the underlying flap (Figure 6).
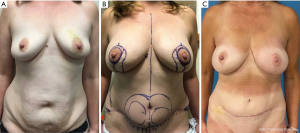
While attention is often focused on optimizing breast symmetry and aesthetics, donor site aesthetics cannot be overlooked. For patients undergoing DIEP flaps, the location of the abdominal scar and umbilicus remains a point of dissatisfaction amongst patients (30). The umbilicus is the focal point of the abdomen and a critical aesthetic landmark, determining the overall cosmetic outcome and patient satisfaction. The approach to umbilical inset should take into consideration abdominal flap thickness and length of the umbilical stalk. Ultimately, the characteristics that define an aesthetically pleasing umbilicus is a vertical orientation, with superior hooding and inferior retraction and slope (31,32). This can be accomplished with an inverted-V umbilicoplasty (33). When appropriate, the inverted-V flap on the abdomen can be anchored to the fascia creating an inferior slope. This method also avoids a circumferential scar which is prone to contracture and can be a distinguishing mark of abdominoplasty. Circumferential defatting of the abdominal flap also creates a natural peri-umbilical indentation. The appropriate location of the umbilicus is a highly debated topic; however, the anterior superior iliac spine is a generally agreed upon reference point (34-36). In our practice, we usually pull the umbilicus slightly upward to separate it from the transverse scar and align it in the midline. In addition to the umbilicus, lower abdominal scar revisions are a common request amongst patients (30). As a result of the shift away from the TRAM flap towards DIEP, harvesting less rectus muscle, rates of abdominal bulge and hernia have decreased significantly, with focus now on optimizing abdominal closure (37). Avoiding step-off deformities and lateral dog-ears are critical in the aesthetic outcome of the abdominal wall. A difference in upper and lower flap thickness can cause a visible step-off. This can be avoided by performing a tapered sub-scarpal resection at time of flap harvest. In the second stage, any residual step off can be liposuction as can any prominence of the mons region to provide an improved aesthetic contour. Lateral dog ears can be revised by direct excision in conjunction with liposuction of the flanks to provide a smooth transition between the area of skin resection and the lateral flank region. For buttock or thigh donor sites, a combination of suctioning and fat grafting can help reduce contour irregularities as seen in Figure 6.
Conclusions
Over the last two decades, breast reconstruction has seen considerable advances, permitting flap success rates of above 99%. As microsurgery becomes standard practice across the country and the number of breast reconstructions continues to rise, the focus appropriately shifts towards patient-centered care. The aesthetics of breast reconstruction is a vital outcome measure that translates directly to patient satisfaction. By understanding the critical elements to restoring a shapely breast, utilizing strategies for optimizing the NAC position, incorporating novel techniques to ensure core projection, and paying attention to the donor site, the skilled microsurgeon can elevate breast reconstruction to the level of true aesthetic surgery where the reconstructed result may surpass the presurgical appearance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ketan M. Patel and Ara A. Salibian) for the series “Advances in Microsurgical Breast Reconstruction” published in Gland Surgery. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-647/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-647/coif). The series “Advances in Microsurgical Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halsted WS. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg 1907;46:1-19. [Crossref] [PubMed]
- Zhang C, Kosiorek H, Hammond JB, et al. The impact of mastectomy and reconstruction technique on patient perceived quality of Life. Am J Surg 2022;224:1450-4. [Crossref] [PubMed]
- Tsangaris E, Klassen AF, Kaur MN, et al. Development and Psychometric Validation of the BREAST-Q Sensation Module for Women Undergoing Post-Mastectomy Breast Reconstruction. Ann Surg Oncol 2021;28:7842-53. [Crossref] [PubMed]
- Wachter T, Del Frari B, Edlinger M, et al. Aesthetic outcomes after surgical repair of pectus excavatum in females: Differences between patients and professional evaluators. Arch Plast Surg 2020;47:126-34. [Crossref] [PubMed]
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg 2009;123:455-62. [Crossref] [PubMed]
- Pirro O, Mestak O, Vindigni V, et al. Comparison of Patient-reported Outcomes after Implant Versus Autologous Tissue Breast Reconstruction Using the BREAST-Q. Plast Reconstr Surg Glob Open 2017;5:e1217. [Crossref] [PubMed]
- Chae MP, Rozen WM, Patel NG, et al. Enhancing breast projection in autologous reconstruction using the St Andrew's coning technique and 3D volumetric analysis. Gland Surg 2017;6:706-14. [Crossref] [PubMed]
- Odobescu A, Keith JN. Preshaping DIEP Flaps: Simplifying and Optimizing Breast Reconstruction Aesthetics. Plast Reconstr Surg 2021;147:1059-61. [Crossref] [PubMed]
- Mayo JL, Allen RJ, Sadeghi A. Four-flap Breast Reconstruction: Bilateral Stacked DIEP and PAP Flaps. Plast Reconstr Surg Glob Open 2015;3:e383. [Crossref] [PubMed]
- Haddock NT, Dickey RM, Perez K, et al. BREAST-Q and Donor Site Comparison in Bilateral Stacked Autologous Breast Reconstruction. Plast Reconstr Surg Glob Open 2022;10:e4413. [Crossref] [PubMed]
- Momeni A, Kanchwala S. Hybrid Prepectoral Breast Reconstruction: A Surgical Approach that Combines the Benefits of Autologous and Implant-Based Reconstruction. Plast Reconstr Surg 2018;142:1109-15. [Crossref] [PubMed]
- Ter Louw RP, Nahabedian MY. Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:51S-9S. [Crossref] [PubMed]
- Hybrid Microsurgical Breast Reconstruction: HyFIL® & HyPAD™ Techniques. Nov 29-Dec 2, 2021, Mar Del Plata, Argentina: Argentinian Society of Plastic, Aesthetic and Reconstructive Surgery 51st National Congress.
- Chung AP, Sacchini V. Nipple-sparing mastectomy: where are we now? Surg Oncol 2008;17:261-6. Erratum in: Surg Oncol 2010;19:114. [Crossref] [PubMed]
- Frey JD, Salibian AA, Levine JP, et al. Incision Choices in Nipple-Sparing Mastectomy: A Comparative Analysis of Outcomes and Evolution of a Clinical Algorithm. Plast Reconstr Surg 2018;142:826e-35e. [Crossref] [PubMed]
- Daar DA, Abdou SA, Rosario L, et al. Is There a Preferred Incision Location for Nipple-Sparing Mastectomy? A Systematic Review and Meta-Analysis. Plast Reconstr Surg 2019;143:906e-19e. [Crossref] [PubMed]
- Chidester JR, Ray AO, Lum SS, et al. Revisiting the free nipple graft: an opportunity for nipple sparing mastectomy in women with breast ptosis. Ann Surg Oncol 2013;20:3350. [Crossref] [PubMed]
- Doren EL, Van Eldik Kuykendall L, Lopez JJ, et al. Free nipple grafting: an alternative for patients ineligible for nipple-sparing mastectomy? Ann Plast Surg 2014;72:S112-5. [Crossref] [PubMed]
- Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [PubMed]
- McKane BW, Korn PT. The fleur-de-lis upper gracilis flap for breast reconstruction: flap design and outcome. Ann Plast Surg 2012;69:383-6. [Crossref] [PubMed]
- Dayan JH, Allen RJ Jr. Lower Extremity Free Flaps for Breast Reconstruction. Plast Reconstr Surg 2017;140:77S-86S. [Crossref] [PubMed]
- Farhadi J, Maksvytyte GK, Schaefer DJ, et al. Reconstruction of the nipple-areola complex: an update. J Plast Reconstr Aesthet Surg 2006;59:40-53. [Crossref] [PubMed]
- Nimboriboonporn A, Chuthapisith S. Nipple-areola complex reconstruction. Gland Surg 2014;3:35-42. [PubMed]
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: A literature review. Eur J Surg Oncol 2016;42:441-65. [Crossref] [PubMed]
- Tousimis E, Haslinger M. Overview of indications for nipple sparing mastectomy. Gland Surg 2018;7:288-300. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Economides JM, Graziano F, Tousimis E, et al. Expanded Algorithm and Updated Experience with Breast Reconstruction Using a Staged Nipple-Sparing Mastectomy following Mastopexy or Reduction Mammaplasty in the Large or Ptotic Breast. Plast Reconstr Surg 2019;143:688e-697e. Erratum in: Plast Reconstr Surg 2019;143:1810-1. [Crossref] [PubMed]
- Stalder MW, Accardo K, Allen RJ, et al. Aesthetic Refinement of the Abdominal Donor Site after Autologous Breast Reconstruction. Plast Reconstr Surg 2015;136:455-61. [Crossref] [PubMed]
- Joseph WJ, Sinno S, Brownstone ND, et al. Creating the Perfect Umbilicus: A Systematic Review of Recent Literature. Aesthetic Plast Surg 2016;40:372-9. [Crossref] [PubMed]
- van Veldhuisen CL, Kamali P, Wu W, et al. Prospective, Double-Blind Evaluation of Umbilicoplasty Techniques Using Conventional and Crowdsourcing Methods. Plast Reconstr Surg 2017;140:1151-62. [Crossref] [PubMed]
- Lesavoy MA, Fan K, Guenther DA, et al. The inverted-v chevron umbilicoplasty for breast reconstruction and abdominoplasty. Aesthet Surg J 2012;32:110-6. [Crossref] [PubMed]
- Dubou R, Ousterhout DK. Placement of the umbilicus in an abdominoplasty. Plast Reconstr Surg 1978;61:291-3. [Crossref] [PubMed]
- Kajikawa A, Ueda K, Katsuragi Y, Kimura S, et al. How to reconstruct a natural and deep umbilicus: three methods of umbilicoplasty for five types of umbilical deformities. Ann Plast Surg 2012;68:610-5. [Crossref] [PubMed]
- Rodriguez-Feliz JR, Makhijani S, Przybyla A, et al. Intraoperative assessment of the umbilicopubic distance: a reliable anatomic landmark for transposition of the umbilicus. Aesthetic Plast Surg 2012;36:8-17. [Crossref] [PubMed]
- Lindenblatt N, Gruenherz L, Farhadi J. A systematic review of donor site aesthetic and complications after deep inferior epigastric perforator flap breast reconstruction. Gland Surg 2019;8:389-98. [Crossref] [PubMed]


