
Clinical outcomes and tumor microenvironment response to radiofrequency ablation therapy: a systematic review and meta-analysis
Highlight box
Key findings
• We identified several proteins and pathways of interest which are important in wound healing, angiogenesis, and cellular growth and survival.
What is known and what is new?
• Radiofrequency ablation (RFA) has been proven safe and effective. However, the exact antitumor molecular mechanism is unknown.
• Several proteins and pathways of interest such as VEGF, AKT, matrix metalloproteinases (MMPs), and c-Met were identified which are implicated in wound healing, angiogenesis, and cellular growth and survival.
What is the implication, and what should change now?
• The identified proteins and pathways of interest may implicate areas of research towards RFA resistance and cancer recurrence.
• Further research may focus on the regulation of these pathways post-RFA to overcome mechanisms of resistance.
Introduction
Background
Radiofrequency ablation (RFA) is a minimally invasive intervention that utilizes a high-frequency thermal current to precisely ablate tumor cells while preserving the surrounding tissue (1). Treatment with RFA is associated with a low complication rate, low mortality, and low cost (2-4). RFA is considered an effective treatment for several cancer types. In the treatment of primary and metastatic lung tumors, RFA is shown to provide adequate ablation margins and preserved pulmonary function (5). RFA as a treatment for hepatocellular carcinoma is favored as it is known to cause minimal complications, including lack of intraabdominal adhesion, and may be considered a survival-enhancing treatment before liver transplantation (6). Researchers have extensively studied RFA of thyroid nodules and continuously proven its efficacy, safety, and cost-effectiveness with an associated long-term follow-up time and reduced tumor volume (7,8).
Rationale and knowledge gap
The exact anti-tumor mechanism of RFA is unknown. An in vivo study on hepatocellular carcinoma reports increased apoptosis and heat shock protein 70 (HSP-70) expression, positively correlated with increased lymphocytic CD8+ T-cell invasion (9). Another study reported the upregulation of the IL-6/c-Met/HGF and VEGF pathways in insufficient RFA of tumor cells (10). Insufficient RFA remains a challenge for tumors larger than 3 cm, oftentimes resulting in recurrence and resistance to further treatment (11,12). Local recurrence rates depend on remaining ablative margins, as recurrence is found to be around the periphery of the ablation zone. Recurrent tumor cells are shown to be increasingly proliferative, neovascularized, and resistant to treatment compared to primary tumor cells (13). Studies have demonstrated the requirement of an additive pharmaceutical therapy to control tumor activity. The addition of a heat shock protein inhibitor was shown to decrease tumor size and increase overall survival in vivo (14). An epidermal growth factor receptor (EGFR) inhibitor was also shown to suppress malignancy in liver tumors in animal models (15).
Objective
Understanding the pathophysiological mechanism of RFA and the created tumor microenvironment may allow researchers to prevent RFA recurrence and resistance. We reviewed the literature for clinical, in vivo, and in vitro studies investigating mechanisms of RFA in various cancers such as breast adenocarcinoma, lung carcinoma, and hepatocellular carcinoma. We identified protein markers and pathway activity present following RFA treatment. We present this article in accordance with the PRISMA reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-555/rc).
Methods
Search strategy
The literature design of this constructed meta-analysis and systematic review was accomplished utilizing the PRISMA guidelines (16). A precise systematic screening was executed using the subsequent electronic scientific databases including EMBASE, PubMed, Web of Science, and Scopus until July 2022. The inclusion criteria of this work were modulated using a sequence of pertinent keywords involving [“radiofrequency ablation”, “radiofrequency thermal ablation”, “RFA”, “ablation”, “incomplete ablation”, “recurrence ablation”, “thermal ablation”, or “insufficient ablation”] and [“antitumor”, “tumor”, “cancer”, “cancerous”, “carcinoma”, “neoplasm”, “tumor destruction”, or “tumorigenesis”] and [“molecular pathway”, “signaling pathway”, “upregulation”, “downregulation”, “recurrence”, or “survival”]. A total of 362 works were identified, with 228 duplicates removed, totaling to 134 remaining works.
Inclusion and exclusion criteria
The designated inclusion criteria were formulated as follows: (I) human, in vivo, or in vitro studies that utilized RFA for tumor tissues; (II) retrospective, observational or diagnostic accuracy design; (III) reported at least one molecular or signaling pathway; (IV) stated any cancer type; (V) reported at least one of the following outcomes: tumor size change, complications, recurrence, or survival; and (VI) no restrictions for age, gender, or geographical localization. On the other hand, the exclusion criteria set was as follows: (I) narrative reviews, literature reviews, editorial materials, letter to editors, or meetings; (II) repetitive or duplicated publications; (III) insufficient or overlap data; and (IV) non-English reports.
Data extraction and manipulation
Two groups of independent researchers (LM, MHH, RE, MH) manually extracted and tabulated the clinical data from the relevant studies. These researchers worked independently to mitigate risk of bias. All studies were reviewed to ensure blinding of participants and researchers and blinding of outcomes to avoid performance and detection bias using the Cochrane Collaboration’s tool for assessing risk of bias. The extracted information was abstracted within a designed tabulated form containing the following items: the surname of the first author, the publication’s date, country/region, study design, the type of cancer, the overall sum of subjects. Moreover, the prognostic findings, survival rate, molecular pathway were reported. Any disagreements raised by the investigators are subjected to resolving by the aid of the third investigator (ET).
Statistical analysis
Wilson Score interval with continuity correction was used for confidence interval calculation. Raw untransformed proportion was reported. To pool quantitative variables, DerSimonian-Laird estimator method was carried out and raw untransformed means were reported. R package ‘metafor’ and ‘meta’ were used. Results showed significant heterogeneity with I2 exceeding 50%, therefore, values of random effects model were selected.
Results
Characteristics of included studies
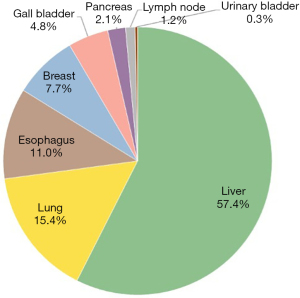
Twenty-two studies with 23 comparisons were eligible (13,14,17-36) (Figure 1). They were published during the period between 2006 and 2022. Of these, 68.5% (N=15) were interventional studies, while others had a retrospective study design (Table 1). All studies were blinded of participants and researchers and blinding of outcomes to avoid performance and detection bias. For all basic science studies, data were de-identified and clinical outcomes were blinded to researchers. The most frequent cancers studied were liver and lung cancers accounting for 57.4% (N=995) and 15.4% (N=267), followed by esophageal (N=190) and breast cancer (N=134) (Figure 2). The studies included 1,732 cancer patients who underwent RFA. Their mean age was 63.06 years (95% CI: 60.57–65.65), and 33% were females (95% CI: 20–49%). They followed up for an average of 33.7 months (95% CI: 22.4–44.9) (Figure 3). Common complications included bleeding in 19% of patients and post-operative pain in 14% of patients, as shown in Figure 4). RFA was administered for an average of 274 seconds (n=14). Insufficient data were available to measure percentage of tumor shrinkage or quantitative recurrence data after RFA. Biopsy of tumor occurred during procedure and patient follow up time was 33 months (n=9). Tumor activity was examined 2 minutes to 72 hours after RFA; changes in molecular environment over time were poorly reported.
Table 1
| Author, year (reference) | Country/region | Study design | Cancer type | Sample size | Mean age, years | Female, % | Mean follow-up, months |
|---|---|---|---|---|---|---|---|
| Kong, 2022 (17) | China | Retro | GI (pancreas, GB, liver) | 150 | 62.2 | 25.3 | 10.7 |
| Wang, 2021 (18) | China | Int | Liver | 120 | 61.0 | 23.3 | 60.6 |
| Li, 2021 (19) | China | Int | Lung | 42 | 58.5 | 81.0 | NA |
| Zhou, 2021 (20) | China | Int | Lung | 25 | NA | NA | NA |
| Zhang, 2020 (13) | China | Int | Liver | 9 | NA | NA | NA |
| Liao, 2020 (21) | Israel | Int | Liver | 216 | NA | NA | NA |
| Wang, 2020 (22) | China | Int | Liver | 8 | NA | NA | NA |
| Tan, 2020 (36) | UK | Retro | Esophagus | 145 | 66.6 | 13.8 | 18.0 |
| Januszewicz, 2020 (23) | UK | Retro | Esophagus | 45 | 67.0 | 15.6 | 49.2 |
| Zhou, 2019 (24) | China | Int | Bladder | 5 | NA | NA | NA |
| Kumar, 2018 (25) | USA | Int | Liver | 145 | NA | NA | NA |
| Abbas, 2017 (26) | UK | Retro | Breast | 11 | 53.0 | 100.0 | 43.0 |
| Hyun, 2016 (27) | South Korea | Retro | Liver | 14 | 63.1 | 14.3 | 45.3 |
| Yang, 2016 (14) | China | Int | Breast | 80 | NA | NA | NA |
| Moussa, 2014 (28) | USA | Int | Liver | 68 | NA | NA | NA |
| Nour-Eldin, 2011 (29) | Germany | Retro | Lung | 200 | 59.7 | 54.0 | NA |
| Chang, 2011 (30) | Taiwan | Int | Liver | 79 | 65.8 | 21.5 | NA |
| Otto, 2010 (32) | Germany | Int | Liver metastasis (CRC) | 28 | 64.0 | 28.6 | 26.7 |
| Dal Bello, 2010 (31) | Italy | Retro | Liver | 207 | 70.0 | 37.2 | 36.0 |
| Solazzo, 2010 (33) | USA | Int | Breast | 43 | NA | NA | NA |
| Nijkamp, 2009 (34) | The Netherlands | Int | Liver metastasis (CRC) | 8 | NA | NA | NA |
| Cho, 2006 (35) | South Korea | Int | Liver | 84 | NA | NA | 16.0 |
Retro, retrospective study; GI, gastrointestinal tumors; GB, gall bladder cancer; Int, intervention study; NA, unknown; CRC, colorectal cancer.


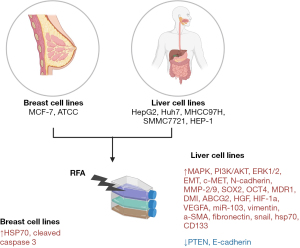
In vitro studies
Nine studies investigated cellular mechanism of RFA on cancer cells (14,18,20,24,36-40). They included 4 types of cancer: breast, liver, lung, and bladder using the cell lines demonstrated in Table 2. Cellular mechanisms of upregulated and downregulated pathways and proteins in vitro are shown in Figure 5.
Table 2
| Author, year (reference) | Country | Cancer | Cell lines | Drug enhancement |
|---|---|---|---|---|
| Wang, 2021 (18) | China | Liver | HepG2, Huh7 | ATRA |
| Zhou, 2021 (20) | China | Lung | NCI-H520, NCI-H226, A549 | Anlotinib |
| Wang, 2021 (40) | China | Lung | nci-h1975 | Osimertinib |
| Jia, 2020 (37) | China | Liver | hepG2 | |
| Tan, 2020 (36) | China | Liver | hepG2 | |
| Zhou, 2019 (24) | China | Bladder | T24, 5637, RT4 | SB431542 - STAT inhibitor |
| Tong, 2017 (38) | China | Liver | MHCC97H, SMMC7721 | |
| Yang, 2016 (14) | China | Liver, breast | MCF-7, HTB-22, ATCC; HEP-1, HTB-52, ATCC | Quercetin – HSP inhibitor |
| Dong, 2013 (39) | China | Liver | SMMC7721, Huh7 | PI3K/Akt inhibitor LY294002, or ERK1/2 inhibitor PD98059 |
RFA, radiofrequency ablation; ATRA, all-trans retinoic acid; STAT, signal transducer and activation of transcription; HSP, heat shock protein.
In vivo studies
Fourteen studies used animal models to investigate the cellular effects of RFA using cell lines from eight liver, three lung, two breast, and one bladder (13,14,18-21,24,25,28,33,34,39-41) (Table 3). Follow-up period was not reported. Cellular mechanisms of upregulated and downregulated pathways and proteins in vivo are shown in Figure 6. Synergistic effect of RFA when combined with some drugs is demonstrated in Table 4.
Table 3
| Author, year (reference) | Country | Cancer | Time (s) | Temp (℃) | Type of animal | No of groups | Total sample size | Injected cell lines |
|---|---|---|---|---|---|---|---|---|
| Wang, 2021 (18) | China | Liver | 300 | 65 | Mice | 2 | 7 | HepG2 |
| Li, 2021 (19) | China | Lung | – | – | Mice | 4 | 42 | C57BL/6 |
| Zhou, 2021 (20) | China | Lung | 120 | 70 | Mice | 4 | 65 | h520, h226 |
| Wang, 2021 (40) | China | Lung | 600 | 47 | Mice | 2 | 20 | BalB/c |
| Qi, 2020 (41) | USA | Liver | 60 | 80 | Mice | 4 | 55 | MTD2 |
| Zhang, 2020 (13) | China | Liver | 600 | 50 | Mice | 3 | NA | HepG2, Huh7 |
| Liao, 2020 (21) | Israel | Liver | 300 | 70 | Mice | NA | 216 | BalB/c, C57BL6 |
| Zhou, 2019 (24) | China | Bladder | 300 | 70 | Mice | 2 | – | BalB/c |
| Kumar, 2018 (25) | USA | Liver | 300 | 70 | Mice, rats | 8 | 142 | C57BL6, F344 |
| Yang, 2016 (14) | China | Breast | 300 | 70 | Rats | 8 | 80 | R3230 |
| Moussa, 2014 (28) | USA | Liver | 300 | 70 | Rats | NA | 68 | R3230 |
| Dong, 2013 (39) | China | Liver | 300 | 47 | Mice | NA | NA | BalB/c |
| Solazzo, 2010 (33) | USA | Breast | 300 | 70 | Rats | 5 | 110 | R3230 |
| Nijkamp, 2009 (34) | The Netherlands | Liver metastasis (CRC) | 50 | – | Mice, rats | 2 | 16 | C26, CC531 |
RFA, radiofrequency ablation; Temp, temperature; CRC, colorectal cancer; NA, not available.
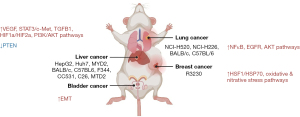
Table 4
| Author, year (reference) | Cancer type | Animal model | Injected cell lines | Drug enhancement | Regulatory effect |
|---|---|---|---|---|---|
| Wang, 2021 (18) | Liver | Mice | HepG2 | ATRA | ATRA triggers apoptosis and suppresses TICs (CD133+ and EpCAM+) by inhibiting PI3K/AKT pathway |
| Li, 2021 (19) | Lung | Mice | C57BL/6 | Melatonin | Analysis of residual tumor after combined treatment revealed depressed activity of MAPK, NF-kappa B, Wnt, and Hedgehog pathways and upregulated P53 pathway in tumors, which was in line with the inhibited tumor growth |
| Zhou, 2021 (20) | Lung | Mice | h520, h226 | Anlotinib | Treatment with anlotinib + RFA inhibited receptor tyrosine kinase or the MPAK/PI3K-AKT pathway kinases |
| Wang, 2021 (40) | Lung | Mice | BalB/c | Osimertinib | OSI inhibited the EGFR/PI3K/AKT pathway and induced apoptosis in thermotolerant NCI-H1975/OSIR cells |
| Qi, 2020 (41) | Liver | Mice | MTD2 | Sunitinib | Sunitinib represses RFA induced PD-1 and PD-L1 upregulation |
| Zhang, 2020 (13) | Liver | Mice | HepG2, Huh7 | Amarogentin | The expression levels of CD133, VEGFA, Dll4, and Notch1 in induced RFA tumor tissues were decreased by amarogentin, and phosphorylated p53 levels were increased |
| Kumar, 2018 (25) | Liver | Mice, rats | C57BL6, F344 | Inhibition of STAT3 with either S3I-201 or micelle-encapsulated curcumin | STAT3 overexpression after RFA led to increase in SAA1, APCS, TIMP1, SERPINAS, CYCLIN D1, P21, SOCS3 |
| Moussa, 2014 (28) | Liver | Rats | R3230 | Bortezomib - HIF-1α inhibitor | Upregulation of HIF-1α after RFA ablation |
| Dong, 2013 (39) | Liver | Mice | BalB/c | PI3K/Akt inhibitor LY294002, or ERK1/2 inhibitor PD98059 | Insufficient RFA may promote the EMT of HCC cells through overexpression of Akt and ERK signaling pathways |
RFA, radiofrequency ablation; EMT, endothelial-mesenchymal transition; HCC, hepatocellular carcinoma.
Literature review of deregulated pathways and markers
Understanding the mechanism of RFA and the created tumor microenvironment may allow researchers to prevent RFA recurrence and resistance. It may help surgeons understand potential complications should the site of the RFA need to undergo further operative treatment, such as increased bleeding due to increased peripheral vascularity as a wound healing mechanism. In this review, we will detail the upregulated pathways in the tumor microenvironment after RFA procedure.
VEGF signaling pathway
As cancerous cells are known to induce angiogenesis, one well-studied pathway in cancer biology is that of the VEGF pathway. In our search, we found four independent studies which found the upregulation and activation of the VEGF pathway following RFA. Most recently, a 2020 US-based study looking at mice with induced hepatocellular cancer treated by RFA were found to have significantly elevated mRNA levels of VEGFA and HIF-1α by quantitative polymerase chain reaction (qPCR) compared to that of non-treated mice. Accordingly, both VEGFR2 and VEGFR1 transcription levels were significantly elevated, with the latter increasing 4-fold. Using sunitinib, an FDA-approved multi-targeted receptor tyrosine kinase (RTK) anti-cancer medication, the authors showed that the therapy was able to downregulate VEGF signaling, decrease VEGFR2 and CD31 (a common marker of vascular differentiation) expression, as well as prolong mice life span and decrease its tumor size post-RFA (41). Another 2020 study analyzing tissue samples of patients with liver cancer who underwent insufficient RFA followed by hepatectomy or solely hepatectomy found increased protein expression of VEGFA in the post-RFA group. Similarly, this study also found a 2-fold increase in CD31 protein expression (13). A distinct work in both rats and mice demonstrated a 2-fold increase in VEGF expression in mice following RFA when compared to mice undergoing a sham procedure (for vehicle control). Interestingly, they found that a STAT3 inhibitor was able to significantly reduce circulating VEGF levels in the RFA-treated mice, yet not to the extent of the sham-treated mice, suggesting that RFA, at least in part, increases VEGF via a STAT3 signaling pathway (25). One final work investigating the VEGF pathway following RFA in two separate animal models (rat and mice) with colorectal liver metastases also found the pathway to be upregulated (34). Interestingly, Nijkamp et al. also analyzed the expression of pro-angiogenic genes in the same animal by analyzing a reference (control) zone and a tissue-ablated zone, and unsurprisingly found no HIF-1α and HIF-2α staining in the reference zone but strong staining for HIF-1α and HIF-2α staining in the ablated zone (34). The Netherlands-based study also found that the tumor-bearing mice five days post-RFA displayed neovascularization with vessels four to five times the size of normal sinusoidal diameter (34). Finally, they found that the usage of a VEGF receptor inhibitor significantly limited post-RFA tumor growth when compared to post-RFA untreated animals (34). Unsurprisingly, the four works analyzing VEGF with respect to RFA for the treatment of cancer all indicate upregulation of the pro-angiogenic pathway. Several anti-VEGF pathways medications have been approved, many of which have been recommended for the treatment of cancers, including renal cell carcinomas and gastrointestinal stromal cancers. Sunitinib, which was able to effectively reduce post-RFA tumor size and prolong mouse life span, as well as other FDA approved medications which possess established safety profiles warrant further study.
AKT signaling pathway
Another studied molecular pathway following RFA is that of the AKT signaling pathway, which is responsible for a multitude of trophic processes including cell proliferation, survival, growth, and metabolism. AKT-pathway mutations have been reported in a multitude of cancers and are specifically associated with less differentiated thyroid carcinomas (42). In 2016, it was found that lung cancer cells following insufficient RFA regrew via PI3K/Akt/HIF-1α signals (43). Since then, three new works (for a total of four studies) have each independently shown the AKT pathway to be upregulated following RFA. Most recently, a 2021 work using a mouse model with hepatocellular carcinomas found three major proteins in the AKT pathway, including p-PI3K, p-AKT, and p-mTOR, to each be significantly upregulated when cells had undergone insufficient RFA. Interestingly, their work demonstrated that the use of all-trans retinoic acid, an FDA approved and well-accepted anti-cancer medication, could normalize the levels of p-PI3K, p-AKT, and p-mTOR to that of the control (primary tumors) as well as suppress tumor regrowth, suggesting a link between the two (18). A separate work in mice with metastatic lung cancer subject to RFA found that residual tumor expressed increased protein levels of AKT, HIF-1α, and CD34 when compared to that of the reference (control) zone which was not ablated. The authors also demonstrated that the use of osimertinib, a signal-targeted therapy against RTKs, could effectively minimize the upregulation of the aforementioned proteins (AKT, HIF-1α, and CD34) as well as significantly decrease tumor growth (40). The final two studies also analyzed the effect of RFA in hepatocellular carcinomas (36,39). One study indirectly found RFA to upregulate the AKT pathway. In both patient tissue samples as well as cultured cell lines, the study demonstrated that miR-103 levels were upregulated in hepatocellular carcinomas of patients undergoing thermal ablation. Then, to demonstrate the link between RFA and the AKT signaling pathway, cells which were treated with miR-103 were found to have upregulated protein expression levels of p-AKT and its downstream AKT-mediators (CyclinD1, p21, Bim, Fasl). Interestingly, the work bolstered the importance of the AKT pathway by analyzing The Cancer Genome Atlas and determined miR-103 upregulation in hepatocellular cancers in comparison to normal patient tissues (36). Finally, one work found that cultured cancer cells treated with insufficient RFA had increased p-AKT and p-ERK1/2 expression compared to non-treated cells. Attractively, they found that though heat-treated cells demonstrated increased proliferation (6.4%), migration (33.2%), and invasion (44.1%) compared to their non-treated counterparts, the effect was completely abated when treated with a specific p-ATK inhibitor, LY294002 (39). Since the AKT pathway mediates a multitude of cancers (including that of the thyroid) and the above-mentioned studies consistently demonstrate the upregulation of the AKT pathway following RFA in hepatocellular carcinomas, medications exploiting the AKT pathway may be of potential interest for patients undergoing RFA of the thyroid.
MMPs
Few studies have analyzed common biomarker proteins following RFA, including the MMP class. The MMPs are a class of endopeptidases which, analogous to a double-edged sword, mediate both the normal and pathological degradation of extracellular matrix proteins. In specific, MMP-2 (digests collagen) and MMP-9 (digests collagen and serves an essential role in leukocyte migration) have been associated with a plethora of cancers and are found circulating at elevated levels in patients with thyroid cancers (24,44). We found three studies investigating MMP-2 and MMP-9, all of which demonstrated elevated transcription or protein levels following RFA in cultured hepatocellular carcinoma cells (14,36,39). One work demonstrated that radiofrequency ablated-patient tissue samples displayed elevated miR-103 levels, and subsequently stimulated recurrent hepatocellular carcinoma cells in vitro with miR-103. They found stimulated-cultured cells had significantly elevated MMP-2 promotor activity as well as elevated MMP-9 levels (36). A separate study directly comparing untreated cells and those treated by RFA also corroborated this notion, finding elevated MMP-2 and MMP-9 protein levels as well (39). The final work examined the effect of the hypoxia-induced environment that RFA inevitably prompts, analyzing hepatocellular cells grown in hypoxic versus normoxic conditions following insufficient RFA. Interestingly, both MMP-2 and MMP-9 protein expression levels were elevated in the post-ablation hypoxic conditions group when compared to the normoxic conditions, strongly suggesting hypoxia-induced MMP-2 and MMP-9 expression (14). By the very nature of their function, MMPs are not merely associated with thyroid cancer overall but also associated with its increased metastasis (45). Considering the above, elucidating the association between MMP-2 and MMP-9 in RFA-ablated thyroid tissue may minimize the risk of thermal-induced malignancy and metastasis.
c-Met (tyrosine-protein kinase Met)
C-Met, also referred to as tyrosine-protein kinase Met, is a protein encoded by the MET gene which is known to play a role in invasive growth, mitogenesis, and morphogenesis (46,47). In specific, c-Met is known to play a role in the development of cancer by activating oncogenic pathways (e.g., PI3K, STAT3) and promote angiogenesis (48). With respect to RFA, we found that c-Met has been moderately studied with a total of four works. The first work utilized human hepatocellular carcinoma cell lines with insufficient heat/RFA and analyzed c-Met at both the transcription and translation levels. Interestingly, they found similar levels of c-Met following RFA sequencing but a significant difference in c-Met expression [using quantitative reverse transcription polymerase chain reaction (RT-qPCR)] between the heat/RFA treated cells and non-treated cells. Similarly, they found that treated and untreated cells had similar c-Met protein levels by immunohistochemistry, but significantly more c-Met expression in the heat/RFA-treated cells upon western blotting. Within the same experiment, they found heat/RFA-treated cells to exhibit higher rates of proliferation, colony formation, and migration than non-treated cells. To ascribe this finding to the c-Met pathway, the authors treated heat/RFA-cells with a c-Met specific inhibitor (SU11274) which was able to effectively neutralize the post-RFA proliferative and colony forming (but not the migratory) effect in both a time-dependent and dose-dependent manner. Finally, with elevated levels of the c-Met specific inhibitor, heat/RFA-treated cells displayed lower proliferation rates than the control (37). Another 2020 study using an intrahepatic metastatic mouse model found that RFA induced metastatic tumor number, cellular proliferation, and intra-tumoral neovascularization when compared to a control sham procedure (needle placement without heating). Treatment with PHA-665752, a specific c-Met inhibitor, reduced aforementioned intrahepatic metastatic parameters (tumor number, cellular proliferation, and intra-tumoral neovascularization) following RFA to that of the control sham procedure group (21). The third work utilized a mouse model with induced hepatocellular cancer treated by RFA to show elevated (~2.5× from control) c-Met mRNA expression in RFA-treated mice which was effectively neutralized in mice treated with both RFA and an FDA-approved multi-targeted RTK drug (sunitinib). RFA with sunitinib treatment, the authors found, could synergistically significantly decrease tumor size and prolong mouse life span (41). Finally, a separate study by Zhou et al. using anlotinib, a RTK inhibitor, combined with RFA could minimize tumor volume more effectively than RFA alone in two separate mouse lung cancer models (lung adenocarcinoma model and lung squamous cell carcinoma model) (20).
Other players
Beyond the major pathways and proteins described above, several genes, proteins, and pathways analyzed with respect to the microenvironment following RFA have yet to be corroborated by further investigation. The following describes the remaining ‘players’. A 2021 work found that mice injected subcutaneously with Lewis lung cancer cells and treated with RFA had elevated Foxo3, ERK2 (2 mediators within the MAPK signaling pathway), and p53 (mediator within the p53 signaling pathway) protein levels. Compared to a sham control group, the authors conversely found decreased protein levels of C-myc and Beta-catenin, two mediators of the Wnt signaling pathway (19). A separate work investigating the use of RFA as a management modality for bladder cancer in lieu of serious radical cystectomy complication found that patient-derived bladder cancer, which had been previously injected into a mouse, tumor volume could be significantly reduced with RFA when compared to a solvent control. Furthermore, they found that synergistic treatment of RFA and SB431542, a specific TGFβ/Smad pathway inhibitor, could significantly reduce tumor volume more than RFA alone, suggesting a growth-promoting signaling pathway inhibition a potential combinatory mechanism against post-RFA tumor re-growth (24). Two works investigated the use of RFA in breast cancer (14,33). Yang et al. showed that RFA along with a heat shock protein inhibitor could slow breast adenocarcinoma tumor growth as well as increase overall survival (26.5±3.4 d) compared to RFA alone (17.6±2.5 d) in a rat model. Their study concluded that HSP70 plays a significant role in the growth of solid breast tumors following RFA (14). The other work compared RFA alone versus intravenous liposomal doxorubicin (an anti-cancer medication) with RFA (combined therapy) on apoptosis-induction in mammary adenocarcinomas. The US-based work found that combination therapy increased cleaved caspase-3 levels (a proxy for apoptosis) more than RFA alone, potentiating chemotherapies, specifically cellular oxidative and nitrative stress inducing therapies, a plausible adjuvant alongside RFA (33). Interestingly, a different work also investigated the synergy of RFA with liposomal doxorubicin (28). Their work found that rats with subcutaneous R3230 tumors treated with RFA increased HIF-1α and HSP70 expression in time and temperature-dependent manners. Attractively, they found that RFA with liposomal doxorubicin significantly reduced HIF-1α rim thickness (125.8±28.2 vs. 48.4±11.8 µm) and total HIF-1α index (48.5±15.9 vs. 6.5±1.4) when compared to RFA alone (28).
Discussion
Key findings
To our knowledge, this study is the most comprehensive analysis of patient outcomes and tumor tissue after a RFA procedure. Our study demonstrated that RFA of tumor tissue is safe and with minimal complications across all tumor types. While complications vary for each type of cancer, such as risk of hoarseness accompanying the risk of RFA of thyroid nodules, bleeding and post-operative pain remain the most common complications, across each cancer type, consistent with our study (49).
Strengths and limitations
Of note, our study had several limitations. Tumor size pre- and post-RFA procedure was not measured. Our study included many types of cancer, all of which exhibit different tumor behavior and therefore potential complications, decreasing the generalizability of our study. Additionally, several data values were unable to be collected, as the research on this given topic is limited. Despite its limitations, our study highlights an area of study that may benefit from robust research activity.
Comparison with other studies
RFA uses thermal energy to destroy tumor tissue in its direct path; however, the intensity of thermal energy decreases at the periphery of the electrode target area. Concern has been raised for a potentially complicated wound healing mechanism in the periphery of the RFA target. Insufficient RFA has been shown to demonstrate recurrence of neoplasms and alteration of normal cellular behavior (50). Suboptimal heat exposure promotes angiogenesis through VEGF pathway by promoting hypoxia-inducible factor-1α and VEGF-α (51). Insufficient RFA has been also shown to upregulate the AKT pathway, promoting neoplastic proliferation and survival through the epithelial-mesenchymal transition pathway, driving cancer metastasis (39). Additional important players found in insufficient RFA of cancer tissue include c-Met, a tyrosine kinase receptor that upregulates embryogenesis, potentially implicating a mechanism that may promote novel neoplastic growth after the RFA procedure, and MMPs, which facilitate wound healing and tissue remodeling following injury, which establish a peripherally reactive cellular environment after RFA (37,52).
Explanation of findings
Decreasing perfusion to target tissue may be a mechanism to decrease the risk of bleeding and hemorrhage such as clipping of vessels, balloon occlusion, or embolization. Risk of injury to adjacent structures is also a concern; studies have monitored temperatures near important structures along the periphery of the ablated tissue to avoid damage (53).
Implications and actions needed
In our review, we outline several pathways and proteins of interest that demonstrate increased angiogenesis, cellular growth, and neoplastic survival. Inhibitors of the aforementioned pathways may be investigated and utilized in further treatment to prevent tumor recurrence after RFA.
Conclusions
The exact anti-tumor mechanism of RFA is unknown, especially in the periphery of the ablated target. Understanding the tumor microenvironment after tumor RFA may help researchers identify mechanisms of resistance. In our review and meta-analysis, we identify several proteins and pathways of interest such as VEGF, AKT, MMPs, and c-Met, of which are important in wound healing, angiogenesis, and cellular growth and survival. Further studies may explore pharmacologic and biologic interventions that may inhibit the pathways to decrease rates of tumor recurrence and growth after RFA.
Acknowledgments
Funding: This work was supported by a research grant from
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gland Surgery for the series “RFA and Recent Innovations in Endocrine Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-555/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-555/coif). The series “RFA and Recent Innovations in Endocrine Surgery” was commissioned by the editorial office without any funding or sponsorship. E.K. served as the unpaid Guest Editor of the series and serves as an Editor-in-Chief of Gland Surgery from May 2017 to April 2024. E.T. receives a research grant (THYROIDGRANT2021-0000000232) from the Bite Me Cancer and facilitated by the American Thyroid Association. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Curley SA, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 2000;232:381-91. [Crossref] [PubMed]
- Froelich MF, Schnitzer ML, Rathmann N, et al. Cost-Effectiveness Analysis of Local Ablation and Surgery for Liver Metastases of Oligometastatic Colorectal Cancer. Cancers (Basel) 2021;13:1507. [Crossref] [PubMed]
- Lorber G, Glamore M, Doshi M, et al. Long-term oncologic outcomes following radiofrequency ablation with real-time temperature monitoring for T1a renal cell cancer. Urol Oncol 2014;32:1017-23. [Crossref] [PubMed]
- Maeda M, Saeki I, Sakaida I, et al. Complications after Radiofrequency Ablation for Hepatocellular Carcinoma: A Multicenter Study Involving 9,411 Japanese Patients. Liver Cancer 2020;9:50-62. [Crossref] [PubMed]
- Picchi SG, Lassandro G, Bianco A, et al. RFA of primary and metastatic lung tumors: long-term results. Med Oncol 2020;37:35. [Crossref] [PubMed]
- Feng K, Ma KS. Value of radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Gastroenterol 2014;20:5987-98. [Crossref] [PubMed]
- Aldea Martínez J, Aldea Viana L, López Martínez JL, et al. Radiofrequency Ablation of Thyroid Nodules: A Long-Term Prospective Study of 24 Patients. J Vasc Interv Radiol 2019;30:1567-73. [Crossref] [PubMed]
- Hussain I, Zulfiqar F, Li X, et al. Safety and Efficacy of Radiofrequency Ablation of Thyroid Nodules-Expanding Treatment Options in the United States. J Endocr Soc 2021;5:bvab110. [Crossref] [PubMed]
- Duan XH, Li TF, Zhou GF, et al. Transcatheter arterial embolization combined with radiofrequency ablation activates CD8(+) T-cell infiltration surrounding residual tumors in the rabbit VX2 liver tumors. Onco Targets Ther 2016;9:2835-44. [Crossref] [PubMed]
- Markezana A, Goldberg SN, Kumar G, et al. Incomplete thermal ablation of tumors promotes increased tumorigenesis. Int J Hyperthermia 2021;38:263-72. [Crossref] [PubMed]
- Cao S, Lyu T, Fan Z, et al. Long-term outcome of percutaneous radiofrequency ablation for periportal hepatocellular carcinoma: tumor recurrence or progression, survival and clinical significance. Cancer Imaging 2022;22:2. [Crossref] [PubMed]
- Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197:728-36. [Crossref] [PubMed]
- Zhang Y, Zhang Y, Wang J, et al. Amarogentin Inhibits Liver Cancer Cell Angiogenesis after Insufficient Radiofrequency Ablation via Affecting Stemness and the p53-Dependent VEGFA/Dll4/Notch1 Pathway. Biomed Res Int 2020;2020:5391058. [Crossref] [PubMed]
- Yang W, Cui M, Lee J, et al. Heat shock protein inhibitor, quercetin, as a novel adjuvant agent to improve radiofrequency ablation-induced tumor destruction and its molecular mechanism. Chin J Cancer Res 2016;28:19-28. [Crossref] [PubMed]
- Su T, Huang M, Liao J, et al. Insufficient Radiofrequency Ablation Promotes Hepatocellular Carcinoma Metastasis Through N6-Methyladenosine mRNA Methylation-Dependent Mechanism. Hepatology 2021;74:1339-56. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Kong YL, Zhang HY, Liu CL, et al. Improving biliary stent patency for malignant obstructive jaundice using endobiliary radiofrequency ablation: experience in 150 patients. Surg Endosc 2022;36:1789-98. [Crossref] [PubMed]
- Wang S, Liu J, Wu H, et al. All-trans retinoic acid (ATRA) inhibits insufficient radiofrequency ablation (IRFA)-induced enrichment of tumor-initiating cells in hepatocellular carcinoma. Chin J Cancer Res 2021;33:694-707. [Crossref] [PubMed]
- Li M, Hao B, Zhang M, et al. Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer metabolism reprogramming and inhibition of multiple pulmonary tumor development. Signal Transduct Target Ther 2021;6:330. [Crossref] [PubMed]
- Zhou W, Gao Y, Tong Y, et al. Anlotinib enhances the antitumor activity of radiofrequency ablation on lung squamous cell carcinoma. Pharmacol Res 2021;164:105392. [Crossref] [PubMed]
- Liao H, Ahmed M, Markezana A, et al. Thermal Ablation Induces Transitory Metastatic Growth by Means of the STAT3/c-Met Molecular Pathway in an Intrahepatic Colorectal Cancer Mouse Model. Radiology 2020;294:464-72. [Crossref] [PubMed]
- Wang XD, Peng JB, Zhou CY, et al. Potential therapies for residual hepatoblastoma following incomplete ablation treatment in a nude mouse subcutaneous xenograft model based on lncRNA and mRNA expression profiles. Oncol Rep 2020;43:1915-27. [Crossref] [PubMed]
- Januszewicz W, Subhash VV, Waldock W, et al. The utility of a methylation panel in the assessment of clinical response to radiofrequency ablation for Barrett’s esophagus. EBioMedicine 2020;58:102877. [Crossref] [PubMed]
- Zhou HQ, Liu MS, Deng TB, et al. The TGF-β/Smad Pathway Inhibitor SB431542 Enhances The Antitumor Effect Of Radiofrequency Ablation On Bladder Cancer Cells. Onco Targets Ther 2019;12:7809-21. [Crossref] [PubMed]
- Kumar G, Goldberg SN, Gourevitch S, et al. Targeting STAT3 to Suppress Systemic Pro-Oncogenic Effects from Hepatic Radiofrequency Ablation. Radiology 2018;286:524-36. [Crossref] [PubMed]
- Abbas H, Erridge S, Sodergren MH, et al. Breast cancer liver metastases in a UK tertiary centre: Outcomes following referral to tumour board meeting. Int J Surg 2017;44:152-9. [Crossref] [PubMed]
- Hyun D, Cho SK, Shin SW, et al. Treatment of Small Hepatocellular Carcinoma (≤2 cm) in the Caudate Lobe with Sequential Transcatheter Arterial Chemoembolization and Radiofrequency Ablation. Cardiovasc Intervent Radiol 2016;39:1015-22. [Crossref] [PubMed]
- Moussa M, Goldberg SN, Kumar G, et al. Radiofrequency ablation-induced upregulation of hypoxia-inducible factor-1α can be suppressed with adjuvant bortezomib or liposomal chemotherapy. J Vasc Interv Radiol 2014;25:1972-82. [Crossref] [PubMed]
- Nour-Eldin NE, Naguib NN, Tawfik AM, et al. Outcomes of an algorithmic approach to management of pneumothorax complicating thermal ablation of pulmonary neoplasms. J Vasc Interv Radiol 2011;22:1279-86. [Crossref] [PubMed]
- Chang ML, Lin SM, Yeh CT. HURP expression-assisted risk scores identify prognosis distinguishable subgroups in early stage liver cancer. PLoS One 2011;6:e26323. [Crossref] [PubMed]
- Dal Bello B, Rosa L, Campanini N, et al. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res 2010;16:2157-66. [Crossref] [PubMed]
- Otto G, Düber C, Hoppe-Lotichius M, et al. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 2010;251:796-803. [Crossref] [PubMed]
- Solazzo SA, Ahmed M, Schor-Bardach R, et al. Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology 2010;255:62-74. [Crossref] [PubMed]
- Nijkamp MW, van der Bilt JD, de Bruijn MT, et al. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg 2009;249:814-23. [Crossref] [PubMed]
- Cho YK, Rhim H, Ahn YS, et al. Percutaneous radiofrequency ablation therapy of hepatocellular carcinoma using multitined expandable electrodes: comparison of subcapsular and nonsubcapsular tumors. AJR Am J Roentgenol 2006;186:S269-74. [Crossref] [PubMed]
- Tan WK, Ragunath K, White JR, et al. Standard versus simplified radiofrequency ablation protocol for Barrett’s esophagus: comparative analysis of the whole treatment pathway. Endosc Int Open 2020;8:E189-95. [Crossref] [PubMed]
- Jia G, Li F, Tong R, et al. c-Met/MAPK pathway promotes the malignant progression of residual hepatocellular carcinoma cells after insufficient radiofrequency ablation. Med Oncol 2020;37:117. [Crossref] [PubMed]
- Tong Y, Yang H, Xu X, et al. Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion. Cancer Sci 2017;108:753-62. [Crossref] [PubMed]
- Dong S, Kong J, Kong F, et al. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Transl Med 2013;11:273. [Crossref] [PubMed]
- Wang J, Ling X, Zhou M, et al. Thermal treatment decreases resistance to osimertinib in non-small cell lung cancer through the EGFR/PI3K/AKT pathway. Neoplasma 2021;68:535-45. [Crossref] [PubMed]
- Qi X, Yang M, Ma L, et al. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J Immunother Cancer 2020;8:e001038. [Crossref] [PubMed]
- Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 2011;7:569-80. [Crossref] [PubMed]
- Wan J, Wu W, Chen Y, et al. Insufficient radiofrequency ablation promotes the growth of non-small cell lung cancer cells through PI3K/Akt/HIF-1α signals. Acta Biochim Biophys Sin (Shanghai) 2016;48:371-7. [Crossref] [PubMed]
- Komorowski J, Pasieka Z, Jankiewicz-Wika J, et al. Matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases and angiogenic cytokines in peripheral blood of patients with thyroid cancer. Thyroid 2002;12:655-62. [Crossref] [PubMed]
- Tian X, Cong M, Zhou W, et al. Relationship between protein expression of VEGF-C, MMP-2 and lymph node metastasis in papillary thyroid cancer. J Int Med Res 2008;36:699-703. [Crossref] [PubMed]
- Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev 2008;27:85-94. [Crossref] [PubMed]
- Johnson M, Koukoulis G, Matsumoto K, et al. Hepatocyte growth factor induces proliferation and morphogenesis in nonparenchymal epithelial liver cells. Hepatology 1993;17:1052-61.
- Zhang Y, Xia M, Jin K, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer 2018;17:45. [Crossref] [PubMed]
- Nemcek AA. Complications of radiofrequency ablation of neoplasms. Semin Intervent Radiol 2006;23:177-87. [Crossref] [PubMed]
- Ke S, Ding XM, Kong J, et al. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma. J Transl Med 2010;8:73. [Crossref] [PubMed]
- Kong J, Kong J, Pan B, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS One 2012;7:e37266. [Crossref] [PubMed]
- Jiang AN, Liu JT, Zhao K, et al. Specific Inhibitor of Matrix Metalloproteinase Decreases Tumor Invasiveness After Radiofrequency Ablation in Liver Tumor Animal Model. Front Oncol 2020;10:561805. [Crossref] [PubMed]
- Diehn FE, Neeman Z, Hvizda JL, et al. Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol 2003;14:1569-76. [Crossref] [PubMed]



