
Cardiac exposure in left-sided breast cancer patients undergoing deep inspiratory breath hold radiation therapy
Highlight box
Key findings
• DIBH enables a considerable reduction of radiation therapy doses to the heart.
• ΔHH is an independent predictor of DIBH benefit.
What is known and what is new?
• DIBH have showed the dosimetric benefits to the heart and cardiac substructures.
• We showed that anatomic characteristics correlate with cardiac doses and may inform patient selection for DIBH.
What is the implication, and what should change now?
• This study may contribute to optimizing of RT in breast cancer patients. Prospective clinical trials are warranted to confirm our findings.
Introduction
Post-operative radiation therapy (PRT) for breast cancer (BC) has been shown to considerably reduce the risk of locoregional recurrence and death (1-3). PRT includes the breast or chest wall, and in patients with high-risk features-additionally lymph node areas (4). Patients with operable BC carry a relatively good prognosis with 5-year overall survival (OS) in the range of 80–90% (5). Thus, late side effects from treatment may be highly relevant. One of the most common long-term health hazards of PRT is cardiovascular disease (CVD) (6). Notably, the dose administered to cardiac substructures is associated with cardiac events (6,7). In left-sided BC, a relevant dose can be received by the left ventricle (LV) and coronary arteries (8), given the close proximity of the heart to the target volume. Hence, left-sided BC patients are at higher risk of late-onset CVD (9). For example, in the study of Darby et al., the risk of major coronary events increased linearly with the mean dose (Dmean) to the heart, at a rate of 7.4% per 1 Gy (10). Nilsson et al. demonstrated that the left anterior descending coronary artery (LAD) dose is strongly associated with the risk of high-grade coronary artery stenosis in left-sided BC patients (11). Alternatively, studies showed the mean heart dose and doses to cardiac substructures to be associated with elevated cardiac enzymes. Skyttä et al. found a positive correlation between Dmean to cardiac substructures and the cardiac troponin T serum level (12). The percentage volume of the LV receiving ≥2 Gy correlated significantly with NT-proBNP (13).
Techniques accounting for breathing movements, such as deep inspiration breath hold (DIBH) and inspiration breath hold (IBH), minimize unnecessary lung and heart RT doses (14,15). During DIBH and IBH, the lungs are inflated, the respiratory motion is minimized, and the heart position changes, leading to a lower exposure than with free breathing (FB) (16). However, breath-holding techniques are associated with additional time, costs, and a high workload. It is, therefore, essential to identify patients who may mostly benefit from them.
This study aimed to retrospectively examine the plausibility, feasibility, and potential benefits of DIBH, and to identify anatomical variables identifying patients likely to benefit most from this procedure. For this purpose, we compared RT doses to normal tissues in left-sided BC patients irradiated using DIBH and FB, and investigated the association between several anatomic features and dose exposure to the heart. We present this article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-160/rc)
Methods
Patients
We retrospectively analyzed the data including the parameters of CT planning scan images and Radiation plan for 67 left-sided BC consecutive patients from The Second Affiliated Hospital of Guangxi Medical University who received RT after breast-conserving surgery (BCS) or mastectomy. Due to the retrospective study design, informed consent was not required. The study was approved by The Second Affiliated Hospital of Guangxi Medical University Ethics Committee (approval No. 2023-KY0003). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Clinical staging and molecular subtype of all patients followed the Guidelines of the Chinese Society of Clinical Oncology (17). Three days before the CT simulation, patients in the DIBH group were trained by a physical therapist to hold their breath. The CT scans started after the chest had stabilized at the maximum height. A minimal breath-hold duration of at least 30 seconds was considered suitable (18). FB patients maintained smooth breathing during scanning. Real-time position management (RPM; Varian Medical Systems, Palo Alto, CA, USA) was used to measure the patients’ respiration. An overview of the detailed procedure is shown in Figure 1.

Dosimetric assessment
The target regions and organs at risk (OARs), including heart, lungs, LAD, LV, and right ventricle (RV), were contoured according to the Radiation Therapy Oncology Group guidelines (19). In patients who underwent mastectomy, RT volumes included the chest wall, while, in patients after BCS, target volumes comprised the whole breast. Regional lymph nodes were irradiated in patients with high-risk features. All patients received 3-dimensional conformal RT. The Eclipse treatment planning system (TPS; Eclipse version 13.6, Varian Medical Systems) was used for contouring and planning. The prescribed dose was defined as 50 Gy in 25 fractions to the planning target volume. Additionally, patients after BCS received a 10 Gy sequential boost dose to the tumor bed. Before RT delivery, weekly cone-beam computed tomography was performed to verify position and examine the treatment reproducibility. The doses to the heart, LV, RV, and LAD were analyzed using the Dmean and maximum dose (Dmax). Additionally, a DVH was created to evaluate the dosimetric parameters of 5 and 20 Gy to the ipsilateral lung and 25 Gy to the heart.
Anatomic and treatment characteristics
According to previous studies, the study parameters anatomic variables were selected from the treatment scan fields (20,21). The chest depth (CD), heart chest wall length (HCWL), and heart chest wall distance (HCWD) were calculated (showed in Figure 1). And the distance between the ipsilateral lung and breast (DBIB) was defined as the distance between the mass centers of ipsilateral lung and the PTV breast (21). The heart height (HH), the heart and ipsilateral lung volumes were also analyzed in both groups. The difference between values and the average value of FB groups were denoted with “∆”, for example, ∆HH.
Statistical analysis
Statistical analyses were performed using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). For all analyses, a P value of <0.05 was considered statistically significant. The t-test, U-test, and chi-squared test were performed to compare the variables between the groups. Pearson’s test was used for correlation analysis for differences between values in two groups and dose parameters (Dmean and Dmax). Relationships between anatomic variables and protective dose reduction (20%) were analyzed. Receiver operator characteristic (ROC) curve analyses were used for predicting parameter thresholds.
Results
Baseline characteristics
The study group included 67 patients (32 managed with DIHB and 35 with FB) treated between June 2017 and December 2019. The median age of the DIBH and FB groups was 48.5 years (range, 27–66 years) and 52 years (range, 35–73 years), respectively. The baseline characteristics, including age, clinical stage, histopathological grade, type of RT field, and systemic adjuvant treatment, were not different between the groups (Table 1).
Table 1
| Characteristics | Total (n=67) | DIBH group (n=32) | FB group (n=35) | P value |
|---|---|---|---|---|
| Age (years), median [range] | 49.0 [27–73] | 48.5 [27–66] | 52 [35–73] | 0.082 |
| Clinical stage, n (%) | ||||
| I | 6 (9.0) | 2 (6.3) | 4 (11.4) | |
| II | 25 (37.3) | 15 (46.9) | 10 (28.6) | |
| III | 30 (44.8) | 12 (37.5) | 18 (51.4) | 0.403 |
| IV | 6 (9.0) | 3 (9.4) | 3 (8.6) | |
| Histopathological grade, n (%) | ||||
| 1–2 | 32 (47.8) | 15 (46.9) | 17 (48.6) | |
| 3 | 12 (17.9) | 4 (12.5) | 8 (22.9) | 0.443 |
| Unknown | 23 (34.3) | 13 (40.6) | 10 (28.6) | |
| Vascular invasion, n (%) | ||||
| Yes | 17 (25.4) | 8 (25.0) | 9 (25.7) | 0.946 |
| No | 50 (74.6) | 24 (75.0) | 26 (74.3) | |
| Lymph node metastasis, n (%) | ||||
| Yes | 49 (73.1) | 24 (75.0) | 25 (71.4) | 0.742 |
| No | 18 (26.9) | 8 (25.0) | 10 (28.6) | |
| Neoadjuvant chemotherapy, n (%) | ||||
| Yes | 16 (23.9) | 6 (18.8) | 10 (28.6) | 0.346 |
| No | 51 (76.1) | 26 (81.3) | 25 (71.4) | |
| Adjuvant chemotherapy, n (%) | ||||
| Yes | 61 (91.0) | 31 (96.9) | 30 (85.7) | 0.242 |
| No | 6 (9.0) | 1 (3.1) | 5 (14.3) | |
| Targeted therapy, n (%) | ||||
| Yes | 28 (41.8) | 16 (50.0) | 12 (34.3) | 0.193 |
| No | 39 (58.2) | 16 (50.0) | 23 (65.7) | |
| Endocrine therapy, n (%) | ||||
| Yes | 34 (50.7) | 17 (53.1) | 17 (48.6) | 0.710 |
| No | 33 (49.3) | 15 (46.9) | 18 (51.4) | |
| RT, n (%) | ||||
| Breast/chest wall only | 5 (7.5) | 2 (6.3) | 3 (8.6) | |
| Breast/chest wall and lymph nodes, except IMC | 46 (68.7) | 23 (71.8) | 23 (65.7) | 0.853 |
| Breast/chest wall and lymph nodes, including IMC | 16 (23.8) | 7 (21.9) | 9 (25.7) | |
| Boost, n (%) | ||||
| No | 59 (88.1) | 29 (90.6) | 30 (85.7) | 0.809 |
| Yes | 8 (11.9) | 3 (9.4) | 5 (14.3) |
DIBH, deep inspiratory breath hold; FB, free breathing; RT, radiation; IMC, internal mammary chain.
Radiation dose distribution to OARs and cardiac substructures
The Dmean values in the DIBH group were lower than in the FB group for the heart (4.68 vs. 6.69 Gy, P<0.001), LAD (12.28 vs. 20.06, P=0.000), LV (4.71 vs. 7.76 Gy, P<0.001), and RV (5.66 vs. 8.67 Gy, P=0.000), corresponding to a decrease by 30.0%, 38.7%, 39.3%, and 34.7%, respectively. The Dmax values in the DIBH group were also lower than in the FB group for the heart (38.19 to 45.28 Gy, P<0.001), LAD (45.30 to 50.85 Gy, P=0.000), LV (31.37 to 44.03 Gy, P=0.000), and RV (35.96 to 49.96 Gy, P<0.001), corresponding to decrease by 15.6%, 10.9%, 28.8%, and 28.0%, respectively. Patients in the DIBH group also showed a significantly lower heart V25 than those in the FB group (2.60% and 6.23%, respectively, P=0.008). In contrast, the Dmean, V5, and V20 of the ipsilateral lung did not differ significantly between the groups (Table 2).
Table 2
| Parameter | DIBH group (median) | FB group (median) | Reduction | P value |
|---|---|---|---|---|
| Left lung mean dose (Gy) | 14.13 | 13.76 | +2.6% | 0.461 |
| Left lung V20 (%) | 24.19 | 24.68 | −2.0% | 0.470 |
| Left lung V5 (%) | 58.90 | 56.92 | +3.4% | 0.247 |
| Right lung mean dose (Gy) | 1.33 | 1.20 | +9.8% | 0.224 |
| Heart mean dose (Gy) | 4.68 | 6.69 | −30.0% | <0.001* |
| Heart max dose (Gy) | 38.19 | 45.28 | −15.6% | <0.001* |
| Heart V25 (%) | 2.60 | 6.23 | −58.2% | 0.008* |
| LAD mean dose (Gy) | 12.28 | 20.06 | −38.7% | 0.000* |
| LAD max dose (Gy) | 45.30 | 50.85 | −10.9% | 0.000* |
| LV-mean (Gy) | 4.71 | 7.76 | −39.3% | <0.001* |
| LV-max (Gy) | 31.37 | 44.03 | −28.8% | 0.000* |
| RV-mean (Gy) | 5.66 | 8.67 | −34.7% | 0.000* |
| RV-max (Gy) | 35.96 | 49.96 | −28.0% | <0.001* |
*, significant differences. DIBH, deep inspiratory breath hold; FB, free breathing; LAD, left anterior descending coronary artery; LV, left ventricle; RV, right ventricle.
Anatomical characteristics
DIBH, compared with FB, resulted in higher ipsilateral lung volume (1,671 vs. 1,022 cc, P<0.001), HH (8.59 vs. 7.28 cm, P<0.001), DBIB (12.73 vs. 10.78 cm, P<0.001), CD (19.97 vs. 19.18 cm, P=0.052), and HCWD (1.57 vs. 1.35 cm, P=0.004) (Table 3). DIBH decreased the heart volume (505 vs. 558 cc, P=0.010) and HCWL (3.26 vs. 3.93 cm, P<0.001). The different value of HH, DBIB, CD, HCWL, and HCWD between DIBH and FB groups were 1.31, 1.95, 0.79, −0.67, and 0.22 cm, respectively.
Table 3
| Parameter (CT-based) | DIBH group (median) | FB group (median) | Reduction | P value |
|---|---|---|---|---|
| HH (cm) | 8.59 | 7.28 | 1.31 cm | <0.001* |
| DBIB (cm) | 12.73 | 10.78 | 1.95 cm | <0.001* |
| CD (cm) | 19.97 | 19.18 | 0.79 cm | 0.052 |
| HCWL (cm) | 3.26 | 3.93 | −0.67 cm | <0.001* |
| HCWD (cm) | 1.57 | 1.35 | 0.22 cm | 0.004* |
| Heart volume (cc) | 505 | 558 | −53 cc | 0.010* |
| Ipsilateral lung volume (cc) | 1,671 | 1,022 | 649 cc | <0.001* |
*, significant differences. CT, computed tomography; HH, heart height; DBIB, the distance between ipsilateral lung and breast; CD, chest depth; HCWL, heart chest wall length; HCWD, heart chest wall distance; DIBH, deep inspiratory breath hold; FB, free breathing.
Correlations between anatomic characteristics and organ at risk doses
ΔHH, ΔDBIB, and ΔHCWL between the FB and DIBH correlated with Heart, LAD, LV and RV Dmean and Dmax values (Table 4). A clear negative correlation was observed between these parameters and ΔHH, ΔDBIB, and ΔHCWL (P<0.05). In contrast, ΔHCWD correlated only with the heart Dmean (P<0.05) and not with other dose-volume parameters (P>0.05).
Table 4
| Variable | ΔHH | P value | ΔDBIB | P value | ΔHCWD | P value | ΔHCWL | P value |
|---|---|---|---|---|---|---|---|---|
| Heart | ||||||||
| Dmean | −0.72 | <0.001* | −0.52 | 0.000* | −0.26 | 0.037* | −0.45 | 0.000* |
| Dmax | −0.61 | <0.001* | −0.37 | 0.002* | −0.13 | 0.284 | −0.45 | 0.000* |
| LAD | ||||||||
| Dmean | −0.52 | 0.000* | −0.40 | 0.000* | −0.19 | 0.118 | −0.26 | 0.033* |
| Dmax | −0.58 | <0.001* | −0.39 | 0.001* | −0.22 | 0.080 | −0.31 | 0.012* |
| LV | ||||||||
| Dmean | −0.65 | <0.001* | −0.65 | <0.001* | −0.21 | 0.088 | −0.40 | 0.001* |
| Dmax | −0.53 | 0.000* | −0.29 | 0.016* | −0.23 | 0.058 | −0.39 | 0.001* |
| RV | ||||||||
| Dmean | −0.49 | 0.000* | −0.39 | 0.001* | −0.14 | 0.277 | −0.32 | 0.008* |
| Dmax | −0.55 | 0.000* | −0.51 | 0.000* | −0.23 | 0.062 | −0.31 | 0.012* |
*, significant differences. Dmean, mean dose; Dmax, maximum dose; LAD, left anterior descending coronary artery; LV, left ventricle; RV, right ventricle; HH, heart height; DBIB, the distance between ipsilateral lung and breast; HCWD, heart chest wall distance; HCWL, heart chest wall length.
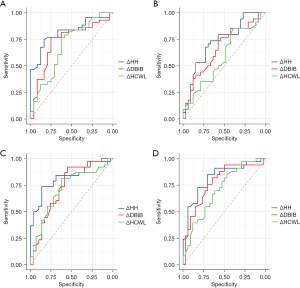
We further constructed the ROC curve to evaluate the predictive value of ΔHH, ΔDBIB, and ΔHCWL for >20% Dmean reduction. The highest efficiency was shown for ΔHH, with the area under the curve (AUC) values of 0.818, 0.725, 0.821, and 0.820 for heart, LAD, LV, and RV Dmean, respectively (all statistically significant; Table 5 and Figure 2).
Table 5
| Parameter | ΔHH | ΔDBIB | ΔHCWL | |||||
|---|---|---|---|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | |||
| Heart Dmean | 0.818 | 0.717, 0.919 | 0.729 | 0.597, 0.860 | 0.685 | 0.545, 0.824 | ||
| LAD Dmean | 0.725 | 0.602, 0.848 | 0.664 | 0.533, 0.796 | 0.585 | 0.446, 0.724 | ||
| LV Dmean | 0.821 | 0.720, 0.922 | 0.735 | 0.607, 0.862 | 0.715 | 0.587, 0.842 | ||
| RV Dmean | 0.820 | 0.717, 0.924 | 0.774 | 0.658, 0.890 | 0.705 | 0.580, 0.830 | ||
Dmean, mean dose; LAD, left anterior descending coronary artery; LV, left ventricle; RV, right ventricle; HH, heart height; DBIB, the distance between ipsilateral lung and breast; HCWL, heart chest wall length; AUC, the area under the curve; CI, confidence interval.
Discussion
Breast cancer patients have benefited from advanced radiation technologies through improved treatment outcomes and longer survival times. However, quality of life (QoL) problems caused by Side-effects should not be overlooked. Thus, DIBH application for reducing cardiac irradiation deserves further investigation.
In this study, DIBH allowed better separation of the heart and LAD to Radiation field, and reduced the irradiated heart volume. We showed that DIBH reduces the Dmean and Dmax to the heart, LAD, LV, and RV. We found that ΔHH has a strong correlation with cardiac mean dose reduction. These results may inform patient selection for DIBH.
Some studies have showed the benefit of DIBH for reducing the mean and maximum dose to the heart and to the LAD (22,23). However, previous studies demonstrated that patients do not benefit equally from DIBH (24). Owing to the complexity of this procedure, it is essential to select patients who will benefit most from it. The predictive value of anatomical and volume parameters for heart exposure was also shown in some studies, including CD, maximum heart depth, tumor bed site, and heart volume in the radiation field (20,25). We demonstrated that out of various analyzed anatomical measures, ΔHH was the best and independent predictor of the Dmean to the heart, LAD, LV, and RV. Hence, this simple measure may identify patients necessitating effective heart protection.
A limitation of breath-holding techniques is their reproducibility, which may affect clinical outcomes (26). Current approaches to overcome this problem include surface-guided radiotherapy, RPM, and image-guided radiation therapy (27,28). DIBH also requires specific training and patient compliance, therefore, treatment efficacy with this technique may be variable. The predictive value of anatomical parameters for heart exposure dose has been highly regarded. This study offered information on potential utility of predictors for patients benefiting from DIBH. We studied some similar research (29,30). The investigation of comparing those parameters from FB and DIBH scans in some patients worthy of further study.
Our study has a few limitations. First, it was retrospective, conducted in a single center, and included patients who underwent both mastectomy and BCS. Additionally, some patients were administered nodal RT, including internal mammary lymph node RT, which increases cardiac exposure. Due to small patient samples, we could not perform stratified analyses considering these variables. Second, the doses to the OARs in each fraction were less strictly controlled than in prospective studies. Currently, advanced RT techniques, such as intensity-modulated RT and volumetric-modulated arc therapy, have been employed in DIBH planning. However, we have identified a reliable predictor for the DIBH benefit. It is imperative, however, that these observations are confirmed within prospective studies.
Conclusions
DIBH significantly reduces cardiac doses in left-sided BC patients undergoing RT. ΔHH may help select patients who will benefit most from DIBH. Future prospective studies are warranted to determine more robustly the dosimetric and clinical benefits of DIBH.
Acknowledgments
Funding: This work was supported by The Basic Ability Enhancement Program for Young and Middle-aged Teachers of Guangxi (No. 2020KY03036), Guangxi Medical University Foundation (No. GXMUYSF201918), Foundation of Guangxi Health and Family Planning Commission (No. Z20190581), and the Foundation of the Second Affiliated Hospital of Guangxi Medical University (No. hbrc202104).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-160/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-160/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-160/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-160/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Second Affiliated Hospital of Guangxi Medical University Ethics Committee (approval No. 2023-KY0003) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Cao L, Xu C, Yi P, et al. Asparaginyl endopeptidase (AEP) regulates myocardial apoptosis in response to radiation exposure via alterations in NRF2 activation. Am J Cancer Res 2021;11:1206-25. [PubMed]
- Schubert LK, Gondi V, Sengbusch E, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol 2011;100:241-6. [Crossref] [PubMed]
- Roumeliotis M, Long K, Phan T, et al. Including internal mammary lymph nodes in radiation therapy for synchronous bilateral breast cancer: an international survey of treatment technique and clinical priorities. Breast Cancer Res Treat 2018;171:471-5. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Banfill K, Giuliani M, Aznar M, et al. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J Thorac Oncol 2021;16:216-27. [Crossref] [PubMed]
- Gee HE, Moses L, Stuart K, et al. Contouring consensus guidelines in breast cancer radiotherapy: Comparison and systematic review of patterns of failure. J Med Imaging Radiat Oncol 2019;63:102-15. [Crossref] [PubMed]
- Taylor C, McGale P, Brønnum D, et al. Cardiac Structure Injury After Radiotherapy for Breast Cancer: Cross-Sectional Study With Individual Patient Data. J Clin Oncol 2018;36:2288-96. [Crossref] [PubMed]
- Bergom C, Bradley JA, Ng AK, et al. Past, Present, and Future of Radiation-Induced Cardiotoxicity: Refinements in Targeting, Surveillance, and Risk Stratification. JACC CardioOncol 2021;3:343-59. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol 2012;30:380-6. [Crossref] [PubMed]
- Skyttä T, Tuohinen S, Boman E, et al. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol 2015;10:141. [Crossref] [PubMed]
- Piroth MD, Baumann R, Budach W, et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther Onkol 2019;195:1-12. [Crossref] [PubMed]
- Bergom C, Currey A, Desai N, et al. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front Oncol 2018;8:87. [Crossref] [PubMed]
- Latty D, Stuart KE, Wang W, et al. Review of deep inspiration breath-hold techniques for the treatment of breast cancer. J Med Radiat Sci 2015;62:74-81. [Crossref] [PubMed]
- Shim JG, Kim JK, Park W, et al. Dose-Volume Analysis of Lung and Heart according to Respiration in Breast Cancer Patients Treated with Breast Conserving Surgery. J Breast Cancer 2012;15:105-10. [Crossref] [PubMed]
- Li JB, Jiang ZF. Chinese Society of Clinical Oncology Breast Cancer Guideline version 2021: updates and interpretations. Zhonghua Yi Xue Za Zhi 2021;101:1835-8. [PubMed]
- Yamauchi R, Mizuno N, Itazawa T, et al. Dosimetric evaluation of deep inspiration breath hold for left-sided breast cancer: analysis of patient-specific parameters related to heart dose reduction. J Radiat Res 2020;61:447-56. [Crossref] [PubMed]
- Li XA, Tai A, Arthur DW, et al. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG Multi-Institutional and Multiobserver Study. Int J Radiat Oncol Biol Phys 2009;73:944-51. [Crossref] [PubMed]
- Register S, Takita C, Reis I, et al. Deep inspiration breath-hold technique for left-sided breast cancer: An analysis of predictors for organ-at-risk sparing. Med Dosim 2015;40:89-95. [Crossref] [PubMed]
- Lin H, Liu T, Shi C, et al. Feasibility study of individualized optimal positioning selection for left-sided whole breast radiotherapy: DIBH or prone. J Appl Clin Med Phys 2018;19:218-29. [Crossref] [PubMed]
- Dumane VA, Saksornchai K, Zhou Y, et al. Reduction in low-dose to normal tissue with the addition of deep inspiration breath hold (DIBH) to volumetric modulated arc therapy (VMAT) in breast cancer patients with implant reconstruction receiving regional nodal irradiation. Radiat Oncol 2018;13:187. [Crossref] [PubMed]
- Misra S, Mishra A, Lal P, et al. Cardiac dose reduction using deep inspiratory breath hold (DIBH) in radiation treatment of left sided breast cancer patients with breast conservation surgery and modified radical mastectomy. J Med Imaging Radiat Sci 2021;52:57-67. [Crossref] [PubMed]
- Ferini G, Valenti V, Viola A, et al. A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients. Cancers (Basel) 2022;14:3477. [Crossref] [PubMed]
- Cao N, Kalet AM, Young LA, et al. Predictors of cardiac and lung dose sparing in DIBH for left breast treatment. Phys Med 2019;67:27-33. [Crossref] [PubMed]
- Kron T, Bressel M, Lonski P, et al. TROG 14.04: Multicentre Study of Feasibility and Impact on Anxiety of DIBH in Breast Cancer Patients. Clin Oncol (R Coll Radiol) 2022;34:e410-9. [Crossref] [PubMed]
- Lu W, Li G, Hong L, et al. Reproducibility of chestwall and heart position using surface-guided versus RPM-guided DIBH radiotherapy for left breast cancer. J Appl Clin Med Phys 2023;24:e13755. [Crossref] [PubMed]
- Rossi M, Laaksomaa M, Aula A. Patient setup accuracy in DIBH radiotherapy of breast cancer with lymph node inclusion using surface tracking and image guidance. Med Dosim 2022;47:146-50. [Crossref] [PubMed]
- Ferini G, Molino L, Tripoli A, et al. Anatomical Predictors of Dosimetric Advantages for Deep-inspiration-breath-hold 3D-conformal Radiotherapy Among Women With Left Breast Cancer. Anticancer Res 2021;41:1529-38. [Crossref] [PubMed]
- S Nair S. A Dosimetric Study Comparing Different Radiotherapy Planning Techniques With and Without Deep Inspiratory Breath Hold for Breast Cancer. Cancer Manag Res 2022;14:3581-7. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


