
Maximizing volume in autologous breast reconstruction: stacked/conjoined free flaps
Introduction
Background
There is an increasing body of literature that reports improved cosmesis, patient satisfaction, and quality of life following autologous breast reconstruction (1-3). With this, the use of autologous tissue continues to grow for women desiring breast reconstruction following mastectomy (4). Autologous breast reconstruction may utilize tissue from a variety of donor sites throughout the body of the patient, including the abdomen, thighs, buttocks and back (5). While these numerous donor sites have been described in the literature, abdominally based free tissue transfer offers numerous advantages and has emerged as the principal donor site for autologous breast reconstruction in women with adequate abdominal tissue volume (4).
Abdominally based tissue breast reconstruction was first reported by Holmstrom when he described moving a free flap involving the rectus abdominis muscle (the transverse rectus abdominis or “abdominoplasty” flap) to reconstruct mastectomy defects (6). Since this development, abdominal based autologous reconstruction has continued to evolve, driven by improved knowledge of angiosomes and perforasomes as well as innovative derivatives of these techniques. Furthermore, the widespread training of plastic surgeons with microsurgical skills has increased the feasibility of performing autologous breast reconstruction at a broader level. Continual refinement of techniques coupled with the desire to meet patients’ evolving demands and expectations has led to constant innovation. One challenge with abdominally based breast reconstruction is addressing patients with larger breasts—particularly in patients with disproportionate or limited available abdominal tissue (7).
Rationale and knowledge gap
The development of stacked/conjoined free flaps is one technique that evolved to address this discrepancy. The primary utility of stacked/conjoined free flaps has been supplementing extra volume for breast reconstruction and providing increased vascularity to free tissue transfers when needed (7). A recent meta-analysis reviewing a total of 26 clinical studies on stacked/conjoined breast reconstruction found that despite patients having a relatively low BMI, the mean combined flap weight for a unilateral breast was over 700 grams (7). Though useful, these stacked/conjoined flaps add more complexity and operative time to already lengthy operations. In this same meta-analysis, operative time averaged nearly 8 hours despite being performed at established microsurgical programs (7). One study has reported that operative times in stacked/conjoined flap reconstruction were significantly higher than single flap breast reconstruction (8). The risks associated with increased operative time and duration of anesthesia should be taken into consideration when performing these cases. While safe outcomes have been well-documented in the literature, there are limited data directly comparing stacked/conjoined flaps and their standard free tissue transfer counterparts (4).
Objective
In this review, we will highlight the use of stacked/conjoined free flaps as well as compile the existing data from the literature on this technique.
Nuances and applications of stacked/conjoined flaps
Considerations for use of stacked/conjoined flaps
When deciding to use a stacked/conjoined free flap for autologous breast reconstruction, several factors must be considered. As with any free flap procedure, preoperative planning is paramount for long-term success. Patients should be encouraged to reach a healthy body weight, which will help to minimize wound healing complications, as well as optimize any chronic vasculopathic diseases (e.g., diabetes mellitus). Further, strict cessation of negative behavioral activities including nicotine use which is known to have adverse effects on wound healing is recommended (9). Additionally, smoking has recently been suggested to increase flap failure in breast reconstruction underscoring the importance of controlling this modifiable risk factor (10).
Certain clinical presentations may also indicate using stacked/conjoined free flaps for autologous breast reconstruction. In massive weight loss patients, it may be challenging to find adequate tissue volume for the desired breast reconstruction using only single free flap techniques due to the abundance of skin but relative paucity of subcutaneous fat. Limited reports have found bilateral stacked free flaps to be well suited for this clinical scenario (11). Alternatively in patients with a higher body mass index (BMI), Sultan et al. reports that unilateral, conjoined, bipedicle deep inferior epigastric perforator (DIEP) flap breast reconstruction can be performed safely in overweight patients with no statistically significant differences in the overall incidence of major or minor complications between the high BMI and low BMI groups (12).
Most importantly, a stacked/conjoined free flap requires adequate vascular inflow and outflow to the flaps at the recipient site to successfully support the larger mass of tissue. Two options for anastomosing the flaps involve either joining the flap pedicles in “parallel” via anterograde and retrograde perfusion from two separate sets of internal mammary vessels (Figure 1) (13-16) or connecting the flap pedicles in “series” via anterograde perfusion from only one internal mammary pedicle with direct anastomosis from the pedicle branch of the first flap to a pedicle branch of the second flap (17). There are challenges to both of these commonly used options. When joining the flaps in “parallel”, it is not always possible to find two adequate sets of vessels for anastomosis, in addition to the increase operative time required for finding and dissecting free a second set of vessels. While interflap anastomoses in “series” require just one set of recipient vessels at the recipient site, it has been a less commonly preferred option compared to using the antegrade and retrograde internal mammary vessels by several authors due to perceived increased risk of flap complication (13,17,18). Metanalysis data, however, reveals no increased risks of any flap complications based on the choice of recipient vessels, though data is limited and more rigorous studies are required to ultimately assess this clinical question (7). Though the internal mammary vessels are commonly used, other options are available for anastomosis including appropriately sized internal mammary perforators, branches of the thoracodorsal, thoracoacromial, and lateral thoracic vessels. Other vascular considerations should be taken account for when planning including pedicle length and caliber. Flaps from the thighs may have either a smaller vessel diameter [profunda artery perforator (PAP) flaps] creating a mismatch with recipient internal mammary vessels or have a shorter pedicle length (gracilis flaps) which may limit flap positioning in the breast pocket (7).
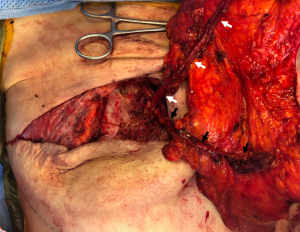
Described options for stacked/conjoined flaps
There are various combinations of abdominal-based stacked/conjoined flaps that have been described in the literature (Table 1) (7). Importantly, the majority of data supporting the use of these flaps are small retrospective series or case reports from single institutions with limited comparative data. Nevertheless, early evidence supporting the safety and feasibility of stacked/conjoined flaps when indicated is promising (7). While the abdomen can be the sole donor site in stacked/conjoined flap unilateral breast reconstruction, bilateral breast reconstruction often necessitates the use of other donor sites. While the more commonly reported flap options for autologous breast reconstruction will be subsequently discussed, it should be noted that donor sites for stacked/conjoined flaps can be selected based on available donor sites and associated volume in conjunction with the patient’s tolerance for donor site morbidity.
Table 1
| Authors | Year | Number | Reconstruction laterality | Flap combo 1 | Flap combo 2 | Flap combo 3 | Flap combo 4 | Flaps lost |
|---|---|---|---|---|---|---|---|---|
| Eltahir et al. | 2022 | 7 patients | Bilateral | DIEP/PAP | 1 | |||
| Murota et al. | 2022 | 1 patient | Bilateral | DIEP/LAP | 0 | |||
| Nakamura et al. | 2022 | 1 patient | Unilateral | SIEA/SIEA | 0 | |||
| Haddock et al. | 2022 | 79 patients | Bilateral | DIEP/PAP | – | |||
| Roggio et al. | 2022 | 7 patients | Unilateral | DIEP/TUG | 0 | |||
| Yoo et al. | 2022 | 2 patients | Bilateral | DIEP/PAP | 0 | |||
| Martinez et al. | 2021 | 28 patients | Bilateral | DIEP/PAP | 0 | |||
| Haddock et al. | 2021 | 2 patients | Bilateral | DIEP/LAP | 0 | |||
| Jo et al. | 2022 | 11 patients | Unilateral | PAP/PAP | 0 | |||
| Haddock et al. | 2021 | 50 patients | Bilateral | DIEP/PAP | msTRAM/PAP | 5 | ||
| Tielemans et al. | 2021 | 1 patient | Unilateral | PAP/PAP | 1 | |||
| Yu et al. | 2020 | 1 patient | Unilateral | DIEP/SIEA | 0 | |||
| Teotia et al. | 2020 | 153 patients | Unilateral, bilateral | DIEP/DIEP | PAP/PAP | DIEP/PAP | 5 | |
| Haddock et al. | 2019 | 388 patients | Unilateral, bilateral | PAP/PAP | DIEP/PAP | DIEP/SIEA | 3 | |
| Tessler et al. | 2019 | 8 patients | Unilateral | LAP/LAP | 0 | |||
| Haddock et al. | 2019 | 20 patients | Unilateral | PAP/PAP | 0 | |||
| Beugels et al. | 2018 | 49 patients | Unilateral, bilateral | DIEP/SCIP | DIEP/SIEA | DIEP/LAP | 2 | |
| Haddock et al. | 2017 | 42 breasts | Unilateral, bilateral | DIEP/PAP | PAP/GAP | PAP/PAP | 2 | |
| Parra | 2017 | 1 patient | Unilateral | PAP/PAP | 0 | |||
| Haddock et al. | 2017 | 21 breasts | Unilateral, bilateral | DIEP/PAP | PAP/GAP | PAP/PAP | 2 | |
| Angrigiani et al. | 2016 | 14 patients | Unilateral | TAP/TAP | 0 | |||
| Rozen et al. | 2016 | 1 patient | Bilateral | DIEP/TUG | 0 | |||
| Patel et al. | 2016 | 25 patients | Unilateral | DIEP/DIEP | 1 | |||
| Stalder et al. | 2016 | 53 patients | Unilateral, bilateral | DIEP/DIEP | PAP/PAP | DIEP/GAP | DIEP/PAP | 5 |
| Malata et al. | 2015 | 25 patients | Unilateral | DIEP/DIEP | 0 | |||
| Mayo et al. | 2015 | 20 patients | Bilateral | DIEP/PAP | 0 | |||
| Park et al. | 2015 | 5 patients | Unilateral | TUG/TUG | 1 | |||
| Koolen et al. | 2015 | 28 patients | Unilateral, bilateral | DIEP/DIEP | DIEP/SIEA | DIEP/DCIA | DIEP/SCIA | 0 |
| Murray et al. | 2015 | 15 patients | Unilateral | DIEP/SIEA | 0 | |||
| Blechman et al. | 2013 | 1 patient | Unilateral | PAP/PAP | 0 | |||
| DellaCroce et al. | 2011 | 55 patients | Unilateral | DIEP/DIEP | 0 | |||
| Chan et al. | 2010 | 1 patient | Unilateral | DIEP/DIEP | 0 | |||
| Figus et al. | 2007 | 1 patient | Unilateral | DIEP/SIEA | 0 | |||
| Ali et al. | 2002 | 1 patient | Unilateral | DIEP/DIEP | 0 | |||
| Spear et al. | 1994 | 10 patients | Unilateral | TRAM/TRAM | 0 |
DIEP, deep inferior epigastric perforator; PAP, profunda artery perforator; LAP, lumbar artery perforator; SIEA, superficial inferior epigastric artery; TUG, transverse upper gracilis; msTRAM, muscle sparing transversus rectus abdominis muscle; SCIP, superficial circumflex iliac artery perforator; GAP, gluteal artery perforator; TAP, thoracodorsal artery perforator; DCIA, deep circumflex iliac artery; SCIA, superficial circumflex iliac artery; TRAM, transversus rectus abdominis muscle.
Unilateral breast reconstruction
One safe option for obtaining multiple free flaps for unilateral autologous breast reconstruction is the use of two hemiabdominal flaps as typically raised in superficial inferior epigastric artery (SIEA), DIEP or transversus rectus abdominis muscle (TRAM) reconstructions (Figure 2) (19-21). A single center series of 40 patients (80 flaps) reported by Beahm and Walton had no flap losses with isolated fat necrosis present in three of the 80 total flaps (19). These flaps can be maintained as one contiguous unit of tissue (“conjoined”), or the tissue separated into two distinct flaps (“stacked”) before both being transferred for a unilateral reconstruction. A single center series of 63 patients undergoing unilateral bipedicled, conjoined DIEP flap reconstruction from Seth et al. also reported no flap losses with three operative interventions for flap salvage (22). Though both are viable options, “conjoined” flaps can have less flexibility for final contouring and shaping in the breast pocket with the added concern of pedicle kinking in certain inset positions. Thus, regardless of whether the two hemiabdominal flaps are maintained as “conjoined” or “stacked”, some authors describe a preference for maintaining two separate pedicles for anastomosis in the chest (23).
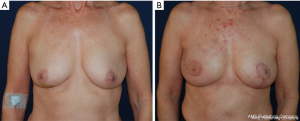
An alternative option would be to maintain the two hemiabdominal flaps as one contiguous unit of tissue based on a single vascular pedicle—sometimes described as “daisy-chaining” (23). With this approach, there is a perceived higher risk of flap loss and/or fat necrosis given decreased vascular diversification (13,17,18,23). If this option is selected, perfusion across the midline must be evaluated intraoperatively using clinical signs and/or angiography, especially in patients with prior abdominal surgeries or history of radiation. Obtaining preoperative computed tomography angiography (CTA) has also proven critical to facilitate intraoperative perforator dissection and provide guidance when performing the anastomoses (24,25). Whether the two hemiabdominal flaps are divided or maintained as contiguous is largely a matter of surgeon preference (13,17,22). The decision may be influenced by the availability of recipient vessels and the need for optimum breast shaping by manipulation of the flaps during inset.
For patients who may not need the volume of two hemiabdominal flaps but need more volume than just one hemiabdominal flap can provide, many other options have been described that both include and do not include an abdominal flap as part of the configuration. Roggio et al. described successfully stacking DIEP and transverse upper gracilis (TUG) flaps for unilateral breast reconstruction in seven patients (26), and multiple reports describe successfully stacking DIEP and SIEA free flaps for a patient requiring unilateral autologous breast reconstruction (27-29). Blechman et al. first reported the successful use of stacked PAP flaps for unilateral breast reconstruction in a patient with Poland syndrome (30), and Jo, Jeon and Han later confirmed in a series of 11 patients that PAP flaps are a viable unilateral reconstructive option in patients with normal-to-low BMIs and limited abdominal tissue (31). In single-institution series of 14 patients, Angrigiani et al. first reported the use stacked thoracodorsal artery perforator flaps with acceptable functional deficit of the donor site and aesthetically acceptable final scarring in all patients (32). Tessler et al. first demonstrated the use of stacked lateral thigh perforator flaps in eight patients (16 flaps) with 100 percent flap survival (33). Recently, a case report from Nakamura et al. has even reported using a bipedicled stacked SIEA flap with deep inferior epigastric artery and vein grafts to extend one of the pedicles, giving further flexibility in using bilateral SIEA flaps for unilateral breast reconstruction (34).
Bilateral breast reconstruction
Finding adequate volumes of tissue for bilateral autologous reconstruction is more challenging since the volume from the two hemiabdominal flaps must be divided amongst the bilateral chests. Numerous options have been proposed as strategies for supplementing tissue volume in augmenting the volume of these autologous reconstructions. One option that maintains a single donor site uses tissue lateral to the hemiabdominal flap that is kept in continuity but raised on its own vascular pedicle, which include the deep circumflex iliac artery, the SIEA, the superficial circumflex iliac artery, or the superior gluteal artery perforators (SGAPs) (35,36). In a study of 49 patients with 90 stacked hemiabdominal extended perforator (SHAEP) flaps, Beugels et al. reported no total flap losses and approximately seven percent of patients experiencing minor complications of fat necrosis, partial flap loss or hematoma (36). In these cases, the additional pedicle can be anastomosed to the primary hemiabdominal flap in series or to the mammary vessels in retrograde fashion as previously discussed.
Donor tissue for bilateral stacked/conjoined free flap breast reconstruction is not limited to the abdomen. While some of the more commonly discussed combinations are listed below, this list is not exhaustive, and any combination of free flaps can theoretically be used to meet the patient’s desired breast volume within the confines of the patient’s anatomy. More commonly described flap options in the literature include the combination of DIEP and PAP flaps (18,37-39), DIEP and lumbar artery perforator (LAP) flaps (40), DIEP and SGAP flaps (41), DIEP and inferior gluteal artery perforator (IGAP) flaps (42), and DIEP and TUG flaps (18,43). When performing four-flap reconstruction, proper consideration should be given to patient positioning and intraoperative efficiency during these lengthy procedures. Given that there may be up to four unique donor sites, patients should be adequately counseled on donor site scar burden, morbidity, and heightened risk for surgical site complications. In order to help facilitate decision making regarding these various flap options, we have constructed a general reconstructive algorithm for addressing these cases (Figure 3).
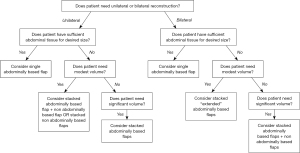
Comparative outcomes
Comparative data between stacked/conjoined and non-stacked/conjoined free flap breast reconstruction are limited and, in some cases, provide conflicting results. The majority of comparative data solely exists for DIEP flap breast reconstruction. A single institution study from Tomouk et al. of 130 patients undergoing DIEP flap breast reconstruction reported that no patients in the bipedicled group developed complications requiring repeat surgery or readmission and that donor site morbidity of bipedicled unilateral DIEP breast reconstructions does not increase compared to unipedicled unilateral and unipedicled bilateral DIEP breast reconstructions (44). However, Xu, Dong and Wang reported in 113 DIEP breast reconstruction patients that overall fat necrosis occurred more frequently in patients receiving bipedicled DIEP reconstruction than those with unipedicled DIEP reconstruction. Despite this finding, more patients in the unipedicled group experienced partial flap loss (45). A 2021 meta-analysis of the available data by Salibian et al. (7) ultimately reported that stacked/conjoined flaps were associated with a lower risk of fat necrosis compared with reconstructions using a single flap, but otherwise there were no differences in flap, breast, or donor-site related complications. This observation of lower rates of fat necrosis may be secondary to the augmented vascular inflow and outflow in stacked/conjoined flaps relative to the amount of tissue thereby reducing the effective area of Holm Zone III tissue in the flap (46). It is important to note that this finding is limited given the high amount of variability in definitions for establishing fat necrosis reported amongst studies necessitating the need for uniformity in reporting (7). Haddock et al. reported in 388 patients with stacked unilateral PAP flaps, DIEP-PAP flaps, or double-pedicle DIEP/SIEA perforator flaps compared to 682 non-stacked/combined DIEP or PAP flaps that the stacked/combined free flap breast reconstruction group had statistically higher deep venous thrombosis rates and take-back rates compared with the non-stacked/combined free flap breast reconstruction group (8). Another study of Haddock et al. reported that BREAST-Q scores in bilateral stacked DIEP-PAP patients demonstrate overall patient satisfaction that is similar to non-stacked bilateral DIEP and non-stacked bilateral PAP reconstruction patients (47). Outside of this report, descriptions of patient-reported outcomes are limited. Salibian et al. identified a lower rate of contralateral symmetrizing reductions in patients undergoing unilateral abdominally based breast reconstruction, which could be an important consideration for patient that like their preoperative breast size and would prefer to maintain its size and shape (4).
Conclusions
Autologous breast reconstruction has experienced many innovations since the “free abdominoplasty” flap was first described by Holmstrom in the 1970s. Performing autologous breast reconstruction with stacked/conjoined free flaps represents another advancement in this field and can be employed as a useful option for patients requiring more tissue volume than can be obtained from a single free flap alone. Though more data, especially comparative studies, are needed for definitive analysis, the use of stacked/conjoined free flaps to increase transferred tissue volume appears to represent a safe and effective tool in the microsurgeon’s armamentarium for treating patients who are otherwise good candidates for autologous breast reconstruction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ketan M. Patel and Ara A. Salibian) for the series “Advances in Microsurgical Breast Reconstruction” published in Gland Surgery. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-577/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-577/coif). The series “Advances in Microsurgical Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. NSK reports that he is on the Board of Directors of the American Society for Aesthetic Plastic Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eltahir Y, Werners LLCH, Dreise MM, et al. Which breast is the best? Successful autologous or alloplastic breast reconstruction: patient-reported quality-of-life outcomes. Plast Reconstr Surg 2015;135:43-50. [Crossref] [PubMed]
- Jeevan R, Browne JP, Gulliver-Clarke C, et al. Surgical Determinants of Patient-Reported Outcomes following Postmastectomy Reconstruction in Women with Breast Cancer. Plast Reconstr Surg 2017;139:1036e-45e. [Crossref] [PubMed]
- Liu C, Zhuang Y, Momeni A, et al. Quality of life and patient satisfaction after microsurgical abdominal flap versus staged expander/implant breast reconstruction: a critical study of unilateral immediate breast reconstruction using patient-reported outcomes instrument BREAST-Q. Breast Cancer Res Treat 2014;146:117-26. [Crossref] [PubMed]
- Salibian AA, Bekisz JM, Frey JD, et al. Comparing outcomes between stacked/conjoined and non-stacked/conjoined abdominal microvascular unilateral breast reconstruction. Microsurgery 2021;41:240-9. [Crossref] [PubMed]
- Opsomer D, van Landuyt K. Indications and Controversies for Nonabdominally-Based Complete Autologous Tissue Breast Reconstruction. Clin Plast Surg 2018;45:93-100. [Crossref] [PubMed]
- Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg 1979;13:423-27. [Crossref] [PubMed]
- Salibian AA, Nolan IT, Bekisz JM, et al. A Systematic Review and Meta-Analysis of Microvascular Stacked and Conjoined-Flap Breast Reconstruction. J Reconstr Microsurg 2021;37:631-42. [Crossref] [PubMed]
- Haddock NT, Cho MJ, Teotia SS. Comparative Analysis of Single versus Stacked Free Flap Breast Reconstruction: A Single-Center Experience. Plast Reconstr Surg 2019;144:369e-77e. [Crossref] [PubMed]
- O'Neill AC, Haykal S, Bagher S, et al. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:1349-55. [Crossref] [PubMed]
- Kalmar CL, Drolet BC, Kassis S, et al. Breast Reconstruction Free Flap Failure: Does Platelet Count Matter? Ann Plast Surg 2022;89:523-8. [Crossref] [PubMed]
- Yoo A, Palines PA, Maier MA, et al. Autologous Breast Reconstruction with Bilateral Stacked Free Flaps in Massive Weight Loss Patients. Plast Reconstr Surg Glob Open 2022;10:e4186. [Crossref] [PubMed]
- Sultan SM, Seth AK, Lamelas AM, et al. Bipedicle-Conjoined Deep Inferior Epigastric Perforator Flaps for Unilateral Breast Reconstruction in Overweight and Obese Patients: Do the Benefits Outweigh the Risks? J Reconstr Microsurg 2020;36:346-52. [Crossref] [PubMed]
- DellaCroce FJ, Sullivan SK, Trahan C. Stacked deep inferior epigastric perforator flap breast reconstruction: a review of 110 flaps in 55 cases over 3 years. Plast Reconstr Surg 2011;127:1093-9. [Crossref] [PubMed]
- Hernandez Rosa J, Sherif RD, Torina PJ, et al. Use of both antegrade and retrograde internal mammary vessels in the bipedicled deep inferior epigastric perforator flap for unilateral breast reconstruction. J Plast Reconstr Aesthet Surg 2017;70:47-53. [Crossref] [PubMed]
- Stalder MW, Lam J, Allen RJ, et al. Using the Retrograde Internal Mammary System for Stacked Perforator Flap Breast Reconstruction: 71 Breast Reconstructions in 53 Consecutive Patients. Plast Reconstr Surg 2016;137:265e-77e. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L, et al. The retrograde limb of internal mammary vessels as reliable recipient vessels in DIEP flap breast reconstruction: a clinical and radiological study. Ann Plast Surg 2015;74:447-53. [Crossref] [PubMed]
- Koolen PG, Lee BT, Lin SJ, et al. Bipedicle-conjoined perforator flaps in breast reconstruction. J Surg Res 2015;197:256-64. [Crossref] [PubMed]
- Mayo JL, Allen RJ, Sadeghi A. Four-flap Breast Reconstruction: Bilateral Stacked DIEP and PAP Flaps. Plast Reconstr Surg Glob Open 2015;3:e383. [Crossref] [PubMed]
- Beahm EK, Walton RL. The efficacy of bilateral lower abdominal free flaps for unilateral breast reconstruction. Plast Reconstr Surg 2007;120:41-54. [Crossref] [PubMed]
- Patel NG, Rozen WM, Chow WT, et al. Stacked and bipedicled abdominal free flaps for breast reconstruction: considerations for shaping. Gland Surg 2016;5:115-21. [PubMed]
- Ali RS, Garrido A, Ramakrishnan V. Stacked free hemi-DIEP flaps: a method of autologous breast reconstruction in a patient with midline abdominal scarring. Br J Plast Surg 2002;55:351-3. [Crossref] [PubMed]
- Seth AK, Koolen PGL, Sultan SM, et al. Unilateral Autologous Breast Reconstruction with Bi-pedicled, Conjoined Deep Inferior Epigastric Perforator Flaps. J Reconstr Microsurg 2019;35:145-55. [Crossref] [PubMed]
- Seth AK, Allen RJ Jr. Modern techniques and alternative flaps in microsurgical breast reconstruction. J Surg Oncol 2018;118:768-79. [Crossref] [PubMed]
- Cho MJ, Haddock NT, Teotia SS. Clinical Decision Making Using CTA in Conjoined, Bipedicled DIEP and SIEA for Unilateral Breast Reconstruction. J Reconstr Microsurg 2020;36:241-6. [Crossref] [PubMed]
- Kim SY, Lee KT, Mun GH. Computed Tomographic Angiography-Based Planning of Bipedicled DIEP Flaps with Intraflap Crossover Anastomosis: An Anatomical and Clinical Study. Plast Reconstr Surg 2016;138:409e-18e. [Crossref] [PubMed]
- Roggio T, Pantelides NM, Morgan M, et al. Stacked TUG and DIEP flaps to reconstruct a single breast: Expanding the scope of autologous breast reconstruction. J Plast Reconstr Aesthet Surg 2022;75:2974-81. [Crossref] [PubMed]
- Yu YH, Ghorra D, Bojanic C, et al. Orienting the superficial inferior epigastric artery (SIEA) pedicle in a stacked SIEA-deep inferior epigastric perforator free flap configuration for unilateral tertiary breast reconstruction. Arch Plast Surg 2020;47:473-82. [Crossref] [PubMed]
- Murray A, Wasiak J, Rozen WM, et al. Stacked abdominal flap for unilateral breast reconstruction. J Reconstr Microsurg 2015;31:179-86. [PubMed]
- Figus A, Fioramonti P, Ramakrishnan V. Stacked free SIEA/DIEP flap for unilateral breast reconstruction in a thin patient with an abdominal vertical midline scar. J Reconstr Microsurg 2007;23:523-5. [Crossref] [PubMed]
- Blechman KM, Broer PN, Tanna N, et al. Stacked profunda artery perforator flaps for unilateral breast reconstruction: a case report. J Reconstr Microsurg 2013;29:631-4. [Crossref] [PubMed]
- Jo T, Jeon DN, Han HH. The PAP Flap Breast Reconstruction: A Practical Option for Slim Patients. J Reconstr Microsurg 2022;38:27-33. [Crossref] [PubMed]
- Angrigiani C, Rancati A, Artero G, et al. Stacked Thoracodorsal Artery Perforator Flaps for Unilateral Breast Reconstruction. Plast Reconstr Surg 2016;138:969e-72e. [Crossref] [PubMed]
- Tessler O, Guste J, Bartow MJ, et al. Stacked Lateral Thigh Perforator Flap as a Novel Option for Autologous Breast Reconstruction. Plast Reconstr Surg 2019;143:1601-4. [Crossref] [PubMed]
- Nakamura R, Tomita K, Omura N, et al. Bipedicled SIEA Flap with Deep Inferior Epigastric Artery and Vein Grafts for Breast Reconstruction. Plast Reconstr Surg Glob Open 2022;10:e4484. [Crossref] [PubMed]
- Malata CM, Rabey NG. Decision Making in Double-Pedicled DIEP and SIEA Abdominal Free Flap Breast Reconstructions: An Algorithmic Approach and Comprehensive Classification. Front Surg 2015;2:49. [Crossref] [PubMed]
- Beugels J, Vasile JV, Tuinder SMH, et al. The Stacked Hemiabdominal Extended Perforator Flap for Autologous Breast Reconstruction. Plast Reconstr Surg 2018;142:1424-34. [Crossref] [PubMed]
- Martinez CA, Fairchild B, Secchi-Del Rio R, et al. Bilateral Outpatient Breast Reconstruction with Stacked DIEP and Vertical PAP Flaps. Plast Reconstr Surg Glob Open 2021;9:e3878. [Crossref] [PubMed]
- Haddock NT, Suszynski TM, Teotia SS. Consecutive Bilateral Breast Reconstruction Using Stacked Abdominally Based and Posterior Thigh Free Flaps. Plast Reconstr Surg 2021;147:294-303. [Crossref] [PubMed]
- Haddock N, Nagarkar P, Teotia SS. Versatility of the Profunda Artery Perforator Flap: Creative Uses in Breast Reconstruction. Plast Reconstr Surg 2017;139:606e-12e. [Crossref] [PubMed]
- Haddock NT, Kelling JA, Teotia SS. Simultaneous Circumferential Body Lift and Four-Flap Breast Reconstruction Using Deep Inferior Epigastric Perforator and Lumbar Artery Perforator Flaps. Plast Reconstr Surg 2021;147:936e-9e. [Crossref] [PubMed]
- Allen RJ, Tucker C Jr. Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg 1995;95:1207-12. [Crossref] [PubMed]
- Allen RJ, Levine JL, Granzow JW. The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg 2006;118:333-9. [Crossref] [PubMed]
- Rozen WM, Patel NG, Ramakrishnan VV. Increasing options in autologous microsurgical breast reconstruction: four free flaps for 'stacked' bilateral breast reconstruction. Gland Surg 2016;5:255-60. [PubMed]
- Tomouk T, Mohan AT, Azizi A, et al. Donor site morbidity in DIEP free flap breast reconstructions: A comparison of unilateral, bilateral, and bipedicled surgical procedure types. J Plast Reconstr Aesthet Surg 2017;70:1505-13. [Crossref] [PubMed]
- Xu H, Dong J, Wang T. Bipedicle deep inferior epigastric perforator flap for unilateral breast reconstruction: seven years' experience. Plast Reconstr Surg 2009;124:1797-807. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. The versatility of the SIEA flap: a clinical assessment of the vascular territory of the superficial epigastric inferior artery. J Plast Reconstr Aesthet Surg 2007;60:946-51. [Crossref] [PubMed]
- Haddock NT, Dickey RM, Perez K, et al. BREAST-Q and Donor Site Comparison in Bilateral Stacked Autologous Breast Reconstruction. Plast Reconstr Surg Glob Open 2022;10:e4413. [Crossref] [PubMed]


