
Factors of perioperative depressive and anxiety symptoms in patients with parotid gland tumor and its influence on postoperative complication and quality of life: a cohort study
Highlight box
Key findings
• Negative emotions have a significant impact on the postoperative complication and quality of life of patients with PGT. According to individual differences, targeted nursing intervention should be supplemented to stabilize the patient’s emotions, improve compliance, and enhance their quality of life.
What is known and what is new?
• Negative emotions have a significant impact on the postoperative complication and quality of life of patients with PGT.
• Apart from negative emotions, there are many independent risk factors for the postoperative quality of life of patients with PGT.
What are the implications, and what should change now?
• During surgical treatment of PGT, clinical efforts should pay attention to patients’ emotions and demeanor, the identification of relevant risk factors as early as possible, the adoption of targeted measures to alleviate the anxiety and depression of patients, and the prevention of complications, so as to improve their prognosis.
Introduction
Parotid gland tumors (PGTs) account for 80% of salivary gland tumors and 2% to 3% of head and neck tumors. Among them, 80% are benign, with the most common being the Warthin tumor and pleomorphic adenoma (1). Malignant tumors of the parotid gland account for about 15% of parotid tumors, with mucinous epidermoid carcinoma being the most common and most highly differentiated type (2). The majority of patients with parotid tumors have no obvious conscious symptoms in the early stage of development, and only some of them experience local pain or enlargement in the parotid area, so the optimal time for diagnosis and treatment is easily missed. Different types of parotid tumors exhibit divergent morbidity characteristics, and the benignity and malignancy of tumors cannot be judged by clinical manifestations and medical history alone but must be combined with ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and other ancillary examinations to make a clear diagnosis (3). In addition, Fine Needle Aspiration Biopsy (FNAB) is the basis of diagnosing salivary gland tumors. In a study by Park et al. (4), a total of 302 patients were diagnosed with parotid cancer after surgery, and 85 of them had no evidence of malignancy before surgery but were confirmed to be malignant by pathology postoperatively. According to the nature, size, location, relationship with facial nerve and patients’ requirements for aesthetics and other different factors of parotid benign tumor, on the premise of ensuring surgical treatment effect, the surgeon should try to make the incision more hidden on the basis of minimizing postoperative complications, such as the “V” incision with concealed postoperative bruises, in order to achieve the best treatment effect of both aesthetics and function. In view of the surgical effect and postoperative aesthetics, it is necessary to explore the balance between the two and develop as far as possible
Surgery is the main treatment method for PGT (5). In recent years, as a new surgical method, endoscopy-assisted resection of benign parotid tumors not only provides sufficient surgical field of view, but also has the advantages of highly aesthetic incision, rapid postoperative recovery and few complications (6). In addition to the surgical resection of malignant tumors, fast neutron radiation is often used in radiation therapy, which is now ranked as the first choice for the radiotherapy of salivary gland malignancies (7). Both benign and malignant PGTs are prone to postoperative complications, such as facial nerve injury, salivary fistula, periauricular sensory abnormalities, Frey syndrome, recurrence, hematoma in the wound, wound infection and First Bite Syndrome (FBS). One study (8) found that the incidence of salivary fistula after parotidectomy can be as high as 14%. In parotid tumor resection surgery, intraoperative injury to the greater auricular nerve is the main cause of abnormal discomfort of peri-auricular and earlobe sensation among patients (9). FBS is a recognized complication after upper neck surgery involving deep lobe of parotid gland (DLP), parapharyngeal space (PPS), or subtemporal fossa (ITF), and its diagnosis is purely clinical and patient history (10). It is typically characterized by the first bite of each meal experiencing sharp pain in the ipilateral parotid gland region, which in severe cases may affect an individual’s ability to tolerate oral eating. According to the results of Xu et al. (11), the incidence of FBS is about 2%. Frey syndrome, also known as auriculotemporal syndrome, refers to symptoms such as transient sweating, flushing, and abnormal sensation of the ipsilateral temporal and anterior ear skin when eating after parotid tumor surgery. Helmus (12) reported that the incidence of Frey syndrome was 1.4% in 146 cases of partial parotidectomy with 10-year follow-up. Another study showed that Frey syndrome occurred in 4–62% of parotidectomized patients between 6 and 18 months after surgery (13-15). It has been suggested that this is due to the regeneration of misaligned postganglionic fibers of the parasympathetic secretory nerve that innervates the parotid gland, causing facial flushing and sweating when the patient eats (16). Poutoglidis et al. showed that the recurrence rate of parotid tumors is as high as 5.7% (17). Inadequate intraoperative resection and rupture during removal are the main causes of the recurrence of benign parotid tumors. Therefore, during the treatment of parotid tumors, we should try to prevent the occurrence of complications intraoperatively, according to different factors such as the nature, size, location, and relationship with the facial nerve of the tumor, and if they occur after surgery, we should intervene as early as possible to achieve early detection and treatment (18).
Benign PGTs generally have a better prognosis, and despite the longer postoperative recovery period, careful care and improved patient compliance can significantly improve patient prognosis. In the process, however, there has been a growing recognition that mental health issues are a growing part of the unmet needs of cancer survivors, including in the form of anxiety and depression, and that mental health can have a significant impact on patient outcomes (19). Because of the special location of parotid gland tumor surgery, postoperative aesthetics often become the focus of attention of patients, and some patients may even have negative emotions. In addition, a study has shown that age, gender, tumor malignancy and other factors are risk factors for negative emotions in cancer patients (20). However, there are few studies on the risk factors for negative emotions in patients with PGTs. The present study aims to analyze the risk factors for negative emotions in the perioperative period and their impact on the prognosis of patients with PGT to provide a reference for improving the prognosis of patients. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-43/rc).
Methods
Research participants
In total, 200 PGT patients admitted to the Affiliated Hospital of Jiangnan University from August 2017 to August 2021 were included in this study. The pathological classification of this study referred to the 2005 histopathological classification of salivary gland tumors (21).
Inclusion criteria: (I) all case data were diagnosed by clear pathological diagnosis, tumor onset site in the parotid area, and complete case data; (II) patients who voluntarily signed the informed consent; (III) parotid tumors were the primary tumors (Figure 1).
Exclusion criteria: (I) patients who were treated after surgery without a clear pathological diagnosis or incomplete information; (II) patients with malignant tumors elsewhere in the body; (III) those with consciousness and communication disorders; (IV) those with psychiatric disorders and patients who were unable to follow medical advice (Figure 1).
The number of patients to be included in this study combined the findings of the previous study group on the incidence of PGTs and the work experience of our researcher, and included all patients with PGTs in the department of stomatology of Affiliated Hospital of Jiangnan University during 5 years. Based on the inclusion and exclusion criteria, a total of 200 cases were proposed to be included. A total of 14 cases were lost due to lost visits, and 186 patients were finally included in this study. All patients received Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) scores 3 days prior to discharge. Patients were followed up 6 months after discharge by outpatient or telephone follow-up, and World Health Organization Quality of Life Scale (WHOQOL) were used to assess the postoperative and quality of life of patients at the last follow-up.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Affiliated Hospital of Jiangnan University (No. LCKY2021132) and informed consent was taken from all the patients.
General information questionnaire
The general information questionnaire included demographic data (gender, age, education level, residency, children or not, spouse or not, etc.) and clinical data (hypertension history, hyperlipidemia history, diabetes history, tumor characteristics, tumor metastasis, intraoperative bleeding, surgery time, hospitalization time, postoperative complications, etc.).
Negative emotion assessment
The SAS was used to reflect the existence and degree of anxiety. This scale consists of 20 items, each rated from 1 to 4. The scores of each item were added together to obtain a crude score and then multiplied by 1.25 to obtain a standard score. The evaluation criteria are SAS score >50, 50–59 for mild anxiety, 60–69 for moderate anxiety, and ≤70 for severe anxiety. The scale has good reliability and validity, and Cronbach’s α coefficients were above 0.75.
The SDS was used to reflect the existence and degree of depression. This scale consists of 20 items, and an SDS score >52 was used as the evaluation criterion. An SDS ≥53 was classified as depression, 53–62 as mild depression, 63–72 as moderate depression, and >72 as severe depression. The SDS has good reliability and validity, and Cronbach’s α coefficients were above 0.75.
Prognostic evaluation
Quality of life evaluation
The quality of life of patients was assessed according to the World Health Organization Quality of Life Scale (WHOQOL) at 6 months after surgery, which includes four dimensions to evaluate patients’ postoperative quality of life, including social domain (SOCIL), environmental domain (ENVIR), psychological domain (PSYCH), and physiological domain (PHYS). The higher the score, the better the quality of life.
The last follow-up visit occurred in February 2022.
Postoperative recurrence
Tumor recurrence was defined as the re-growth of the tumor as primary cancer at or near the same site during the follow-up period. All enrolled patients were followed up for 1 year by telephone, internet, or outpatient service to determine whether the tumor had recurred.
The last follow-up occurred in August 2022.
Statistical analysis
The results of each scale were entered into a computer for score conversion, and statistical analysis was performed using SPSS 26 (IBM SPSS, USA), with measured data expressed as the mean and standard deviation and count data expressed as frequencies and percentages. Statistical analysis between the groups was performed using the t-test and chi-square test, and multiple linear regression analysis was conducted to determine the independent risk factors for negative mood and poor prognosis in patients during the perioperative period. Two-sided P<0.05 was considered statistically significant.
Results
Baseline data
The baseline characteristics of the patients are shown in Table 1. A total of 189 patients with parotid tumors were included in this study, among which 91 (48.92%) were males and 95 (51.08%) were females. Also, 22 patients (11.83%) had malignant tumors and 164 patients (88.17%) had benign tumors. The SAS scores were 50.68±7.45 and 44.27±8.07 in these two groups, respectively, and the SDS scores were 51.95±6.73 and 46.46±7.90, respectively. The patients’ anxiety and depression scores differed significantly between the two groups (P<0.05). A total of 178 patients (95.70%) had spouses and 8 patients (4.30%) had no spouse, while the SAS scores were 44.76±8.10 and 51.13±9.67 in these two groups, respectively, which were statistically different (P<0.05). In addition, a total of 89 patients (47.85%) had high school or higher education and 97 patients (52.15%) had less than high school education. Their SAS scores were 46.96±8.89 and 43.27±7.23, respectively, and their SDS scores were 48.46±7.89 and 45.88±7.84, respectively.
Table 1
| Item | N (%) | SAS | SDS | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | t/F | P | Mean ± SD | t/F | P | |||
| Age (years) | 0.491 | 0.624 | 1.679 | 0.095 | ||||
| ≤40 | 29 (15.59) | 45.72±8.18 | 49.38±7.14 | |||||
| >40 | 157 (84.41) | 44.90±8.28 | 46.69±8.04 | |||||
| Gender | 0.212 | 0.833 | 0.529 | 0.597 | ||||
| Male | 91 (48.92) | 44.90±8.78 | 47.43±8.24 | |||||
| Female | 95 (51.08) | 45.16±7.75 | 46.81±7.69 | |||||
| Benignity or malignancy tumor | 3.748 | 0.001 | 3.112 | 0.002 | ||||
| Benign | 164 (88.17) | 44.27±8.07 | 46.46±7.90 | |||||
| Malignant | 22 (11.83) | 50.68±7.45 | 51.95±6.73 | |||||
| Fertility or not | 0.060 | 0.952 | 1.430 | 0.154 | ||||
| Have children | 180 (96.77) | 45.04±8.24 | 46.96±7.98 | |||||
| No children | 6 (3.23) | 44.83±9.48 | 51.67±5.99 | |||||
| Spouse or not | 2.156 | 0.032 | 1.510 | 0.133 | ||||
| Have spouse | 178 (95.70) | 44.76±8.10 | 46.93±7.76 | |||||
| No spouse | 8 (4.30) | 51.13±9.67 | 51.25±11.22 | |||||
| Education level | 3.116 | 0.002 | 2.238 | 0.026 | ||||
| High school and above | 89 (47.85) | 46.96±8.89 | 48.46±7.89 | |||||
| Below high school | 97 (52.15) | 43.27±7.23 | 45.88±7.84 | |||||
| Residence | 1.391 | 0.166 | 1.082 | 0.281 | ||||
| Countryside | 94 (50.54) | 44.20±8.73 | 46.49±8.03 | |||||
| City | 92 (49.46) | 45.88±7.68 | 47.75±7.86 | |||||
| Hypertension or not | 2.177 | 0.031 | 0.560 | 0.576 | ||||
| Yes | 68 (36.56) | 46.75±8.93 | 47.54±8.27 | |||||
| No | 118 (63.44) | 44.04±7.70 | 46.86±7.78 | |||||
| Hyperlipidemia or not | 0.928 | 0.355 | 0.837 | 0.404 | ||||
| Yes | 78 (41.94) | 44.37±7.49 | 46.54±7.53 | |||||
| No | 108 (58.06) | 45.51±8.76 | 47.53±8.25 | |||||
| Diabetes or not | 1.286 | 0.200 | 0.344 | 0.732 | ||||
| Yes | 69 (37.10) | 46.04±7.95 | 46.86±7.70 | |||||
| No | 117 (62.90) | 44.44±8.40 | 47.26±8.13 | |||||
| Presence of distant metastases or not | 2.158 | 0.032 | 3.229 | 0.001 | ||||
| Yes | 4 (2.15) | 53.75±6.13 | 59.50±7.19 | |||||
| No | 182 (97.85) | 44.84±8.20 | 46.84±7.77 | |||||
SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; SD, standard deviation.
Furthermore, the patients’ anxiety and depression scores were likewise significantly different in both groups (P<0.05). Sixty-eight patients (36.56%) with concurrent hypertension had a SAS score of 46.75±8.93, while 118 patients (63.44%) with no hypertension had a SAS score of 44.04±7.70, which was significantly lower than that in the group with concurrent hypertension (P<0.05). The SAS scores were 53.75±6.13 and 44.84±8.20, respectively, and the SDS scores were 59.50±7.19 and 46.84±7.77 in the two groups, respectively. The anxiety and depression scores were significantly higher in those with distant metastases than in the non-metastatic group (P<0.05). In conclusion, the nature of the tumor, education level, and whether distant metastasis occurred significantly influenced the SAS and SDS scores of patients. In addition, the presence of concurrent hypertension and a spouse also significantly influenced the SAS scores of patients.
Risk factors of patients’ anxiety and depression analyzed by multiple linear regression
Patient’s higher education, those with hypertension, malignant tumors, facial paresis or transient paralysis, and relapse during 1 year of follow-up were independent risk factors for patient anxiety, as shown in Table 2. Meanwhile, high education, tumor metastasis, and Frey syndrome in patients after surgery were independent risk factors for patient depression, as shown in Table 3.
Table 2
| Related factor | B | SE | t | P |
|---|---|---|---|---|
| Malignant tumor | −6.060 | 1.841 | −3.291 | 0.001 |
| No spouse | −3.797 | 2.738 | −1.387 | 0.167 |
| High school and above | 3.552 | 1.086 | 3.271 | 0.001 |
| Presence of distant metastases or not | 0.600 | 4.158 | 0.144 | 0.885 |
| Hypertension | 2.275 | 1.140 | 1.996 | 0.048 |
| Salivary fistula | −2.562 | 2.250 | −1.139 | 0.256 |
| Skewed corners of the mouth | 0.904 | 2.487 | 0.364 | 0.717 |
| Bleeding | −3.143 | 2.820 | −1.114 | 0.267 |
| Facial paresis or transient paralysis | 6.476 | 2.432 | 2.663 | 0.008 |
| Frey syndrome | 2.725 | 1.946 | 1.401 | 0.163 |
| Permanent facial palsy | 5.425 | 2.437 | 2.226 | 0.027 |
| Relapse during 1 year of follow-up | 5.498 | 2.311 | 2.379 | 0.018 |
SAS, Self-Rating Anxiety Scale; SE, standard error.
Table 3
| Related factor | B | SE | t | P |
|---|---|---|---|---|
| Malignant tumor | −3.236 | 1.862 | −1.737 | 0.084 |
| No spouse | −2.308 | 2.769 | −0.834 | 0.406 |
| High school and above | 2.616 | 1.098 | 2.382 | 0.018 |
| Presence of distant metastases or not | 8.530 | 4.205 | 2.028 | 0.044 |
| Salivary fistula | −0.059 | 2.170 | −0.027 | 0.978 |
| Skewed corners of the mouth | 4.500 | 2.432 | 1.850 | 0.066 |
| Bleeding | −2.775 | 2.688 | −1.033 | 0.303 |
| Facial paresis or transient paralysis | 0.697 | 2.349 | 0.297 | 0.767 |
| Frey syndrome | 4.814 | 1.869 | 2.576 | 0.011 |
| Permanent facial palsy | 2.436 | 2.327 | 1.047 | 0.296 |
| Relapse during the 1 year of follow-up | 3.875 | 2.240 | 1.730 | 0.085 |
SDS, Self-Rating Depression Scale; SE, standard error.
Prognostic indicators of patients with parotid tumors
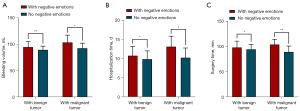
Among the prognostic indicators of patients with benign parotid tumors, there were 45 patients (27.44%) with negative emotions, whose mean bleeding volume was 94.69±10.33 mL, mean hospitalization time was 10.73±2.42 d, and mean operative time was 98.27±12.44 min. Meanwhile, there were 119 patients (72.56%) without negative emotions, whose mean bleeding volume was 89.43±7.07 mL, mean hospitalization time was 9.83±2.18 d, and mean operative time was 94.48±10.04 min, all of which were significantly lower than those in the negative emotion group (P<0.05).
Among the patients with malignant parotid tumors, there were 9 patients (40.91%) with negative emotions, whose mean bleeding volume was 103.33±14.00 mL, mean hospitalization time was 13.08±2.74 d, and mean operative time was 104.00±10.24 min. Meanwhile, there were 13 patients (59.09%) without negative emotions, whose mean bleeding volume was 92.46±9.61 mL, mean hospitalization time was 10.23±2.52 d, and mean operative time was 89.54±11.61 min, all of which were significantly lower than the negative emotion group (P<0.05), as shown in Table 4 and Figure 2.
Table 4
| Item | N (%) | Bleeding volume, mL (mean ± SD) | Hospitalization time, d (mean ± SD) | Surgery time, min (mean ± SD) |
|---|---|---|---|---|
| Patients with benign tumors | ||||
| With negative emotions | 45 (27.44) | 94.69±10.33 | 10.73±2.42 | 98.27±12.44 |
| No negative emotions | 119 (72.56) | 89.43±7.07 | 9.83±2.18 | 94.48±10.04 |
| t | −3.148 | −2.293 | −2.014 | |
| P | 0.003 | 0.023 | 0.046 | |
| Patients with malignant tumors | ||||
| With negative emotions | 9 (40.91) | 103.33±14.00 | 13.008±2.74 | 104.00±10.24 |
| No negative emotions | 13 (59.09) | 92.46±9.61 | 10.23±2.52 | 89.54±11.61 |
| t | −2.168 | −2.408 | −3.083 | |
| P | 0.042 | 0.028 | 0.006 | |
SD, standard deviation.
Complications
In patients with benign PGTs, there were significant differences in the presence or absence of negative emotions (P<0.05) between the two groups of patients with skewed corners of the mouth, facial paresis or transient paralysis, Frey syndrome, permanent facial palsy, and relapse during the 1 year follow-up period, while there were no significant differences in postoperative salivary fistula and bleeding. In patients with malignant PGTs, none of the complications were significantly different between the groups with or without negative emotions, as shown in Table 5.
Table 5
| Item | n (%) | Salivary fistula, n (%) | Skewed corners of the mouth, n (%) | Bleeding, n (%) | Facial paresis or transient paralysis, n (%) | Frey syndrome, n (%) | Permanent facial palsy, n (%) | Relapse during 1 year of follow-up, n (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||||||||
| Patients with benign tumor | |||||||||||||||||||||
| With negative emotions | 45 (27.44) | 1 (2.2) | 44 (97.8) | 6 (13.3) | 39 (86.7) | 1 (2.2) | 44 (97.8) | 6 (13.3) | 39 (86.7) | 8 (17.8) | 37 (82.2) | 6 (13.3) | 39 (86.7) | 7 (15.6) | 38 (84.4) | ||||||
| No negative emotions | 119 (72.56) | 9 (7.6) | 110 (92.4) | 4 (3.4) | 115 (96.6) | 6 (5.0) | 113 (95.0) | 4 (3.4) | 115 (96.6) | 6 (5.0) | 113 (95.0) | 4 (3.4) | 115 (96.6) | 3 (2.5) | 116 (97.5) | ||||||
| χ2 | 1.627 | 5.671 | 0.635 | 5.671 | 6.783 | 5.671 | 9.689 | ||||||||||||||
| P | 0.202 | 0.017 | 0.425 | 0.017 | 0.009 | 0.017 | 0.002 | ||||||||||||||
| Patients with malignant tumor | |||||||||||||||||||||
| With negative emotions | 9 (40.91) | 2 (22.2) | 7 (77.8) | 1 (11.1) | 8 (88.9) | 0 (0.0) | 9 (100.0) | 2 (22.2) | 7 (77.8) | 2 (22.2) | 7 (77.8) | 2 (22.2) | 7 (77.8) | 2 (22.2) | 7 (77.8) | ||||||
| No negative emotions | 13 (59.09) | 1 (7.7) | 12 (92.3) | 0 (0.0) | 13 (100.0) | 1 (7.7) | 12 (92.3) | 0 (0.0) | 13 (100.0) | 2 (15.4) | 11 (84.6) | 0 (0.0) | 13 (100.0) | 1 (7.7 ) | 12 (92.3) | ||||||
| χ2 | 0.953 | 1.513 | 0.725 | 3.178 | 0.167 | 3.178 | 0.953 | ||||||||||||||
| P | 0.329 | 0.219 | 0.394 | 0.075 | 0.683 | 0.075 | 0.329 | ||||||||||||||
WHOQOL and its analysis by multiple linear regression for patients with parotid tumors
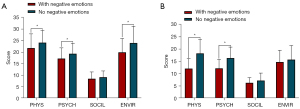
In patients with benign parotid tumors, the physical domain scores were 21.78±6.12 versus 24.14±5.28 for those with and without combined negative emotions; 17.22±4.63 versus 19.33±4.52 in the psychological domain; and 19.93±5.98 versus 23.96±7.25 in the environmental domain, and the differences in these three domains were significantly different (P<0.05), as shown in Table 6 and Figure 3A. In contrast, among patients with malignant PGTs, only the physical and psychological domains differed significantly between patients with and without negative emotions (P<0.05). Their physical domain scores were 12.11±4.01 versus 18.31±5.65, respectively, and their psychological domain scores were 12.22±3.60 versus 16.38±4.46, respectively (Table 6, Figure 3B).
Table 6
| Item | N (%) | PHYS (mean ± SD) |
PSYCH (mean ± SD) |
SOCIL (mean ± SD) |
ENVIR (mean ± SD) |
|---|---|---|---|---|---|
| Patients with benign tumor | |||||
| With negative emotions | 45 (27.44) | 21.78±6.12 | 17.22±4.63 | 8.51±2.93 | 19.93±5.98 |
| No negative emotions | 119 (72.56) | 24.14±5.28 | 19.33±4.52 | 9.14±2.73 | 23.96±7.25 |
| t | 2.447 | 2.645 | 1.298 | 3.320 | |
| P | 0.015 | 0.009 | 0.196 | 0.001 | |
| Patients with malignant tumor | |||||
| With negative emotions | 9 (40.91) | 12.11±4.01 | 12.22±3.60 | 6.33±2.12 | 14.78±4.79 |
| No negative emotions | 13 (59.09) | 18.31±5.65 | 16.38±4.46 | 7.23±3.06 | 15.77±5.70 |
| t | 2.825 | 2.319 | 0.760 | 0.427 | |
| P | 0.010 | 0.031 | 0.456 | 0.674 | |
WHOQOL, The World Health Organization Quality of Life; PHYS, physiological domain; SD, standard deviation; PSYCH, psychological domain; SOCIL, social domain; ENVIR, environmental domain.
Multiple linear regression analysis showed that malignancy, higher literacy, living in an urban area, concurrent hyperlipidemia, prolonged surgery, having negative emotions, and the occurrence of complications were independent risk factors affecting the prognosis of patients (Table 7).
Table 7
| Related factor | B | SE | t | P |
|---|---|---|---|---|
| Age (years) | −0.037 | 0.080 | −0.464 | 0.643 |
| Gender | −1.475 | 1.428 | −1.033 | 0.303 |
| Benign or malignant tumor | 22.277 | 2.541 | 8.767 | 0.000 |
| Fertility or not | 0.090 | 4.320 | 0.021 | 0.983 |
| Spouse or not | 3.103 | 3.528 | 0.879 | 0.380 |
| Education level | −3.537 | 1.443 | −2.450 | 0.015 |
| Residence | −3.197 | 1.434 | −2.230 | 0.027 |
| Hypertension or not | −0.780 | 1.472 | −0.530 | 0.597 |
| Hyperlipidemia or not | −2.959 | 1.443 | −2.051 | 0.042 |
| Diabetes or not | 1.073 | 1.459 | 0.735 | 0.463 |
| Presence of distant metastases or not | 5.855 | 5.487 | 1.067 | 0.288 |
| Bleeding volume | 0.070 | 0.084 | 0.831 | 0.407 |
| Hospitalization time | −0.159 | 0.313 | −0.510 | 0.611 |
| Surgery time | −0.139 | 0.066 | −2.097 | 0.037 |
| With negative emotions or not | −9.068 | 1.784 | −5.084 | 0.000 |
| With complications or not | 3.237 | 1.571 | 2.061 | 0.041 |
WHOQOL, The World Health Organization Quality of Life; SE, standard error.
Discussion
Salivary gland tumors are a more complex type of head and neck tumor, among which approximately 60–80% are located in the parotid gland, which are known as parotid tumors (22,23). In our study, 88% of parotid tumors were benign and 12% were malignant, which is consistent with previous reports (24-26), highlighting that benign parotid tumors currently account for the vast majority of tumors. However, this does not mean that patients do not need to pay attention to them. Different pathological types have varying qualities. For example, the most common pleomorphic adenoma has the potential for malignant transformation and a tendency to recur even after treatment (27). Moreover, regardless of whether the tumor is benign or malignant, it will affect the facial nerve function, facial appearance, and swallowing function, including through transient or permanent facial palsy, skin ulcers, and swallowing disorders, or worse, it will affect the symmetry and aesthetics of the patient’s face. This can easily trigger a strong psychological stress response in patients, resulting in negative emotions such as anxiety and depression. The results of this study found that patients’ education, hypertension, tumor malignancy, postoperative complications including transient or permanent facial palsy, and recurrence were independent risk factors for anxiety, while patients’ education, tumor metastasis, and postoperative Frey syndrome were independent risk factors for depression. The patient’s educational level often determines his or her knowledge of medical science, and the more knowledgeable the patient is about medical science, the more worried he or she is about it, which greatly increases their psychological burden and contributes to negative emotions. Transient or permanent facial palsy and Frey’s syndrome after surgery can mean a loss of physical integrity and reduced aesthetics for the patient. This can be psychologically difficult for patients, who often appear to be restless, fearful, and anxious, and even have a strong psychological stress response, which can have a negative impact on the body. Therefore, according to their psychological condition, medical staff should encourage patients to alleviate their fears and reduce their psychological burden and choose different ways to communicate with patients according to their different levels of understanding of the disease and their different psychological tolerance. This will help to enhance their confidence in treatment, guide them to treat the disease correctly, and actively cooperate with the treatment.
Currently, surgical resection is still the preferred treatment for PGT. Various surgical methods include superficial parotidectomy, partial superficial parotidectomy, total parotidectomy, and radical parotidectomy (28). It has been reported in the literature that the incidence of nerve palsy after superficial parotidectomy is 15–25% and 20–50% after total parotidectomy, while the incidence of permanent facial nerve palsy is 5–10%, and salivary fistula can occur in up to 14% of cases (10,29). McGurk et al. (30) reported incidence rates of 5% and 10% for Frey’s syndrome and transient facial nerve palsy, respectively. A meta-analysis of nine studies (involving a total of 1,882 patients) by Albergotti et al. (31) showed transient facial nerve palsy in 8% of patients and Frey’s syndrome in 4.5% of patients. Together, these findings suggest that complications after parotid tumor surgery are relatively common, which can seriously affect the prognosis of patients. Our study found that the benignity of the tumor, whether the patient had negative emotions, the operative time, and whether there were postoperative complications were independent risk factors affecting the patient’s prognosis. This requires medical staff to protect the important anatomical structures of the maxillofacial region intraoperatively and to avoid postoperative complications as much as possible. For example, the surgeon can adequately dissect and protect the parotid ducts, ligate and remove the branch ducts in the surgical area, and provide adequate postoperative negative pressure drainage to reduce or avoid postoperative salivary fistulae. In addition, they can also fill the defective surgical cavity with fillers to reduce the degree of depression in the surgical area, and establish a barrier between the parasympathetic nerve endings in the parotid gland and the skin and sweat glands to prevent misaligned nerve growth, thereby preventing Frey syndrome.
In addition, negative emotion is also an important factor affecting the patient’s prognosis. According to different factors, such as characteristics of the parotid tumor, its size, location, and relationship with the facial nerve, doctors should try to ensure a more concealed incision based on minimizing postoperative complications while ensuring the surgical treatment effect, so as to achieve the best treatment effect that takes into account both aesthetics and function, thus reducing patients’ psychological burden and enabling them to better cooperate with the rehabilitation treatment.
Our study also found that the recurrence rate of benign parotid tumors was around 2.5–15.6% within 1 year after surgery, which is higher than the 1–5% recurrence rate reported in the literature (32), indicating that although the tumors are benign, they still have a high level of recurrence, probably due to incomplete resection, rupture of the envelope, or satellite lesions. Therefore, to prevent the recurrence of both benign and malignant tumors, physicians should carefully and comprehensively evaluate the size of the mass and confirm the extent of mass excision based on physical examination results and imaging data preoperatively, and also pay attention to the tumor-free principle intraoperatively to prevent tumor rupture and implantation, thereby avoiding tumor recurrence to the greatest extent, improving the postoperative quality of life of patients, and enhancing their prognosis.
The main drawback of this study is that due to limited manpower and time, the follow-up period was short and the prognosis of patients could not be followed up continuously and the postoperative survival rate of the two groups could not be studied. Therefore, we recommend that a longer follow-up period be applied in future studies.
Conclusions
PGT patients are prone to various postoperative complications and still have a high possibility of negative emotions. Clinical efforts should pay attention to patients’ emotions and demeanor, the identification of relevant risk factors as early as possible, the adoption of targeted measures to alleviate patients’ anxiety and depression, and the prevention of complications, so as to improve the prognosis of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-43/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-43/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-43/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Affiliated Hospital of Jiangnan University (No. LCKY2021132) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Larian B. Parotidectomy for Benign Parotid Tumors. Otolaryngol Clin North Am 2016;49:395-413. [Crossref] [PubMed]
- Lee DH, Jung EK, Lee JK, et al. Comparative analysis of benign and malignant parotid gland tumors: A retrospective study of 992 patients. Am J Otolaryngol 2023;44:103690. [Crossref] [PubMed]
- Jin H, Kim BY, Kim H, et al. Incidence of postoperative facial weakness in parotid tumor surgery: a tumor subsite analysis of 794 parotidectomies. BMC Surg 2019;19:199. [Crossref] [PubMed]
- Park H, Han S, Park SJ, et al. Oncological outcomes of preoperatively unexpected malignant tumors of the parotid gland. Eur Arch Otorhinolaryngol 2021;278:2033-40. [Crossref] [PubMed]
- Jeong SH, Kim HY, Lee DH, et al. Facial nerve neurorrhaphy due to unexpected facial nerve injury during parotid gland tumor surgery. Eur Arch Otorhinolaryngol 2020;277:2315-8. [Crossref] [PubMed]
- Dong Y, Zhang J, Li Y, et al. Endoscope-Assisted Resection of Benign Parotid Tumors via Concealed Post-Auricular Sulcus Incision. Laryngoscope 2023;133:133-8. [Crossref] [PubMed]
- Chiodo C, Gros S, Emami B, et al. Intraoperative radiation therapy for locally advanced and recurrent head and neck cancer. Mol Clin Oncol 2022;17:158. [Crossref] [PubMed]
- Mantsopoulos K, Goncalves M, Iro H. Transdermal scopolamine for the prevention of a salivary fistula after parotidectomy. Br J Oral Maxillofac Surg 2018;56:212-5. [Crossref] [PubMed]
- Grammatica A, Perotti P, Mancini F, et al. Great auricular nerve preservation in parotid gland surgery: Long-term outcomes. Laryngoscope 2015;125:1107-12. [Crossref] [PubMed]
- Hayashi K, Onda T, Ogane S, et al. Idiopathic first bite syndrome treated with Rikkosan: a case report. J Oral Maxillofac Surg Med Pathol 2019;31:350-5.
- Xu V, Gill KS, Goldfarb J, et al. First Bite Syndrome After Parotidectomy: A Case Series and Review of Literature. Ear Nose Throat J 2022;101:663-7. [Crossref] [PubMed]
- Helmus C. Subtotal parotidectomy: a 10-year review (1985 to 1994). Laryngoscope 1997;107:1024-7. [Crossref] [PubMed]
- Drummond PD. Mechanism of gustatory flushing in Frey's syndrome. Clin Auton Res 2002;12:144-6. [Crossref] [PubMed]
- Rustemeyer J, Eufinger H, Bremerich A. The incidence of Frey's syndrome. J Craniomaxillofac Surg 2008;36:34-7. [Crossref] [PubMed]
- Neumann A, Rosenberger D, Vorsprach O, et al. The incidence of Frey syndrome following parotidectomy: results of a survey and follow-up. HNO 2011;59:173-8. [Crossref] [PubMed]
- Motz KM, Kim YJ. Auriculotemporal Syndrome (Frey Syndrome). Otolaryngol Clin North Am 2016;49:501-9. [Crossref] [PubMed]
- Poutoglidis A, Tsetsos N, Sotiroudi S, et al. Parotid Gland Tumors in Northern Greece: A 7-year Retrospective Study of 207 Patients. Otolaryngol Pol 2020;75:1-5. [Crossref] [PubMed]
- Chen F, Li Y, Ke X, et al. Application analysis of a modified retroauricular hairline incision in the resection of a benign parotid gland tumor. Hua Xi Kou Qiang Yi Xue Za Zhi 2021;39:293-9. [Crossref] [PubMed]
- Yi Jean C, Syrjala Karen L. Anxiety and Depression in Cancer Survivors. The Medical clinics of North America 2017;101:1099-113. [Crossref] [PubMed]
- Morrison EJ, Novotny PJ, Sloan JA, et al. Emotional Problems, Quality of Life, and Symptom Burden in Patients With Lung Cancer. Clin Lung Cancer 2017;18:497-503. [Crossref] [PubMed]
- Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J 2006;85:74.
- Chang YJ, Huang TY, Liu YJ, et al. Classification of parotid gland tumors by using multimodal MRI and deep learning. NMR Biomed 2021;34:e4408. [Crossref] [PubMed]
- Saravakos P, Kourtidis S, Hartwein J, et al. Parotid Gland Tumors: A Multicenter Analysis of 1020 Cases. Increasing Incidence of Warthin's Tumor. Indian J Otolaryngol Head Neck Surg 2022;74:2033-40. [Crossref] [PubMed]
- Mashrah MA, Al-Sharani HM, Al-Aroomi MA, et al. Surgical interventions for management of benign parotid tumors: Systematic review and network meta-analysis. Head Neck 2021;43:3631-45. [Crossref] [PubMed]
- Inaka Y, Kawata R, Haginomori SI, et al. Symptoms and signs of parotid tumors and their value for diagnosis and prognosis: a 20-year review at a single institution. Int J Clin Oncol 2021;26:1170-8. [Crossref] [PubMed]
- Moore MG, Yueh B, Lin DT, et al. Controversies in the Workup and Surgical Management of Parotid Neoplasms. Otolaryngol Head Neck Surg 2021;164:27-36. [Crossref] [PubMed]
- Stafford ND, Wilde A. Parotid cancer. Surg Oncol 1997;6:209-13. [Crossref] [PubMed]
- Psychogios G, Bohr C, Constantinidis J, et al. Review of surgical techniques and guide for decision making in the treatment of benign parotid tumors. Eur Arch Otorhinolaryngol 2021;278:15-29. [Crossref] [PubMed]
- Massimilla EA, Motta G, Magaldi M, et al. Minimal Margin Surgery and Intraoperative Neuromonitoring in Benign Parotid Gland Tumors: Retrospective Clinical Study. J Pers Med 2022;12:1641. [Crossref] [PubMed]
- McGurk M, Thomas BL, Renehan AG. Extracapsular dissection for clinically benign parotid lumps: reduced morbidity without oncological compromise. Br J Cancer 2003;89:1610-3. [Crossref] [PubMed]
- Albergotti WG, Nguyen SA, Zenk J, et al. Extracapsular dissection for benign parotid tumors: a meta-analysis. Laryngoscope 2012;122:1954-60. [Crossref] [PubMed]
- Ogle OE. Salivary Gland Diseases. Dent Clin North Am 2020;64:87-104. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



