
Preoperative planning of unilateral breast reconstruction with pedicled transverse rectus abdominis myocutaneous (TRAM) flaps: a pilot study of perforator mapping
Highlight box
Key findings
• There was no statistical change in outcome with use of computed tomographic angiography (CTA) planning for pedicled TRAM flap.
What is known and what is new?
• Preoperative CTA has become part of the routine workup in breast reconstruction with deep inferior epigastric artery perforator (DIEP) flaps;
• The role of preoperative CTA for breast reconstruction with the pedicled TRAM flap has not yet been established.
What is the implication, and what should change now?
• Further study and risk/benefit analysis may better highlight the role of CTA in pedicled TRAM flap planning.
Introduction
The pedicled transverse rectus abdominis myocutaneous (TRAM) flap is a well-established autologous reconstructive option for breast defects associated with the surgical treatment of breast cancer (1). It is chosen for its aesthetic outcomes, avoidance of prosthetic implants, short operative times compared with microsurgical reconstruction, low donor site morbidity, high patient satisfaction (2-5) and low cost (6).
The pedicled TRAM flap with its horizontally oriented skin flap was introduced as a variation on the vertical rectus abdominis flap in 1982 by Hartrampf et al. During this time conventional angiography was performed preoperatively in up to 50% of patients to confirm the patency of the communication between the internal mammary artery and deep epigastric vascular systems (7). Vascularity of the myocutaneous flap was also confirmed by intraoperative Doppler ultrasound of rectus muscle pedicle.
Preoperative computed tomographic angiography (CTA) has since become part of the routine workup in breast reconstruction with deep inferior epigastric artery perforator (DIEP) flaps. It allows the surgeon to pre-select the ideal donor site to minimise flap-related complications (8-10), reduce operative time and the overall postoperative morbidity of the reconstruction (11,12). The use of preoperative angiography has enabled significant advances in microsurgical autologous breast reconstruction. However, the role of preoperative CTA for breast reconstruction with the pedicled TRAM flap has not yet been established.
This paper compares a continuous cohort of patients undergoing breast reconstruction with pedicled TRAM flaps without preoperative imaging to a comparable cohort of continuous patients undergoing the same procedure, with the same surgeon, at the same institutions, but with the use of preoperative CTA. The aim of this study is to compare the intra- and post-operative outcomes in patients who underwent pedicled TRAM flap for unilateral breast reconstruction with or without preoperative CTA to determine if this imaging is associated with improved outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-529/rc).
Methods
A cohort study of consecutive patients who underwent unilateral breast reconstruction with pedicled TRAM flaps by a single surgeon was conducted. All eligible patients were included and provided informed consent as required, with all surgery performed at two institutions. Consecutive non-imaged patients all underwent surgery between 2012–2015, and all imaged patients in the immediate period afterwards, between 2015 and 2019. Institutional ethics approval was obtained at both centres: Monash Health (registration No. RES-19-0000-740Q) and Peninsula Health (registration No. ERM # 86700) and this study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patient characteristics and outcome measures were compared between those who underwent preoperative CTA and those without preoperative CTA prior to unilateral breast reconstruction with pedicled TRAM flaps. Medical records of all patients were retrospectively reviewed for patient characteristics and differences in intraoperative and postoperative outcomes between the two groups. Patient characteristics included age, body mass index, smoking status, diabetes, previous abdominal surgery, adjuvant and neoadjuvant therapies were compared.
In the CTA group, the number and size of perforators were collected for each patient. Intraoperative outcomes included choice of contralateral versus ipsilateral TRAM flap, use of abdominal mesh and total operative time (including mastectomy time for immediate cases). Primary postoperative outcomes were flap-related complications including partial flap loss (<50% flap necrosis), total flap loss, and fat necrosis. In this study fat necrosis was defined as any subcutaneous tissue firmness persisting for at least 5 months following surgery. Secondary postoperative outcomes included breast-related complications such as mastectomy flap necrosis, haematoma and infection requiring readmission. Length of hospital stay, unplanned ICU admission and amount of blood transfusions required were also included as secondary outcomes.
There were two primary donor-site related complications included in data collection—the presence of incisional hernia and abdominal bulge. Hernia was defined as any postoperative abdominal wall fascial defect requiring surgical repair and bulge was any protrusion of abdominal wall apparent on clinical examination without an obvious fascial defect. Other donor site outcomes were presence of seroma, haematoma and abdominal wound infection or dehiscence requiring revision surgery. It is noted these complications can occur concurrently in the same patient.
Technique
Prior to mid-2015 without the availability of preoperative CTA, both contralateral and ipsilateral pedicled TRAM flaps were used equally. Between mid-2015 and 2019, all patients underwent preoperative CTA for planning purposes. Key information evaluated was the characteristics of the deep and superior epigastric artery (SEA) and their perforators exiting the rectus abdominis muscles. Characteristics included the presence, number, calibre and course of the artery and their perforators in relation to the rectus abdominis muscle.
The CTA scanning technique comprised:
- Patient positioning during scanning matching the operative position, with no restrictive clothing or bands across the skin of the region being imaged, and minimal table tilt;
- Scan range limited to the region of the body from which the flap may be harvested;
- CTA scanning protocol comprised an arterial phase scan, with bolus tracking technique used to identify filling of the appropriate vessels with contrast as a means to initiate scanning. Using this technique, the scan was timed from the abdominal aorta with a delay of 10–22 s to achieve arterial phase filling;
- The computed tomography (CT) scanners used were Siemens Somatom Sensation 64 multi-detector row CT scanners (Siemens Medical Solutions, Erlangen, Germany);
- Intravenous contrast was used in all cases, with no oral contrast used, and comprised non-ionic iodinated contrast media Omnipaque 350 (Amersham Health, Princeton, USA). Intravenous access was accessed through a cubital fossa vein, with an 18-guage cannula and injection performed with a biphasic power injection pump at a flow rate of 4–6 mL/s;
- Image reformatting software was achieved with Osirix (OsiriX Medical Imaging Software, GPL Licensing Open Source Initiative). Multi-planar three-dimensional reconstructions were achieved with maximum intensity projection (MIP) and volume rendered technique (VRT) reconstructions (Figure 1).
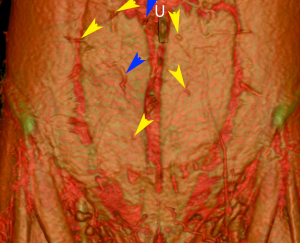
In those without CTAs, hemiabdomen choice was based largely on abdominal scarring from previous procedures. If no scars were present, then most commonly a contralateral TRAM flap was raised as it was thought to provide better contour from the tunnelled pedicle and a longer pedicle length, as described in the literature. In those patients who underwent CTAs, their imaging was used to identify the hemiabdomen with optimal perforators as described below—this was the sole deciding factor for the hemiabdomen of choice. Favourable characteristics were perforators with larger calibre and those situated ideally on the skin paddle for the recipient site—usually in the medial paraumbilical row. Based on this information provided by the CTA the decision was made preoperatively to use either the contralateral or ipsilateral hemiabdomen for reconstruction. The diameter and number of perforators supplying the flap also allowed the surgeon to reliably estimate the size of the flap, incorporating additional Hartrumpf Scheflan zones 2 and 3 as appropriate (7).
Intraoperatively, the knowledge of perforator characteristics allowed the surgeon to target the location of perforators and spare the maximal amount of muscle and rectus sheath. Incision through the rectus sheath was isolated to the site of known perforators and extended minimally to the spare as much rectus sheath as possible. In the group without CTA planning a larger incision along the whole width of the rectus muscle was made and incorporated in the flap to include maximal number of perforators. The rectus sheath was closed in an identical manner in both groups with 1 monofilament nylon.
Statistical analysis
Baseline and demographic characteristics were summarized using descriptive statistics. Pearson’s correlation or Fishers exact test or logistic regression were used to determine significant relationship between preoperative CTA and outcomes.
Results
During the 7-year study period, 34 consecutive patients underwent ipsilateral breast reconstruction with pedicled TRAM flap. Twenty-three of these 34 patients underwent immediate skin and nipple sparing mastectomies. The mean age of the cohort was 58 years (42–75 years) with an average follow-up time of 3.9 years (1 month–7 years). There were 18 patients in the “with CTA” group and 16 patients in the “without CTA” group. There was no statistically significant difference in patient characteristics between the two groups (Table 1), however a greater number of smokers and ex-smokers were present in the “with CTA” group.
Table 1
| Demographics | Without CTA (n=16) | With CTA (n=18) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 57±8 | 58±9 | 0.58 |
| BMI (kg/m2), mean ± SD | 27.7±3.7 | 28.6±5.7 | 0.56 |
| Smoking status, n [%] | 0.51 | ||
| Smoker | 1 [6.3] | 1 [5.6] | |
| Ex-smoker | 6 [37.5] | 6 [33.3] | |
| Diabetes, n [%] | 1 [6] | 2 [11] | 1.0 |
| Previous pregnancy, n [%] | 12 [75] | 14 [78] | 1.0 |
| Previous abdominal surgery, n [%] | 8 [50] | 9 [50] | 1.0 |
| Neoadjuvant chemotherapy, n [%] | 5 [31] | 4 [22] | 0.7 |
| Neoadjuvant radiotherapy, n [%] | 1 [6.3] | 1 [5.6] | 1.0 |
| Adjuvant chemotherapy, n [%] | 5 [31] | 5 [28] | 1.0 |
| Adjuvant radiotherapy, n [%] | 6 [37.5] | 7 [38.9] | 1.0 |
| ASA score, n [%] | 0.41 | ||
| 1 | 3 [19] | 1 [6] | |
| 2 | 9 [56] | 13 [72] | |
| 3 | 4 [25] | 4 [22] |
P values calculated using t-test of Fisher’s exact test. CTA, computed tomographic angiogram; SD, standard deviation; BMI, body mass index; ASA, American Society of Anaesthetists.
There was a higher percentage of contralateral TRAM flap reconstructions performed in the “with CTA” group at 78% compared with 56% in the “without CTA” group (Table 2). The average operative time was comparable between the two groups. The “with CTA” group had lower rate of postoperative ICU admissions (P=0.59). Length of hospital stay was similar between the two groups.
Table 2
| Operative outcomes | Without CTA (n=16) | With CTA (n=18) | P value |
|---|---|---|---|
| Immediate reconstruction, n [%] | 9 [56] | 14 [78] | 0.28 |
| Contralateral TRAM, n [%] | 9 [56] | 14 [78] | 0.27 |
| No. of perforators, mean ± SD | N/A | 2±0.87 | – |
| Size largest perforator (mm), mean ± SD | N/A | 1.4±0.28 | – |
| Contralateral breast reduction, n [%] | 5 [31] | 7 [39] | 0.73 |
| Abdominal mesh use, n [%] | 0 | 0 | – |
| Total operative time (including mastectomy time for immediate cases) (min), mean ± SD | 285±59 | 293±72 | 0.73 |
| Length of hospital stay (days), mean ± SD | 9±2 | 9±6 | 0.83 |
P values calculated using t-test or χ2-test. CTA, computed tomographic angiogram; TRAM, transverse rectus abdominis myocutaneous; SD, standard deviation.
There was no complete flap loss in both groups and comparable rates of partial flap loss between the two groups. Partial flap loss occurred once (6%) in the “without CTA” group while there were two (11%) occurrences in the “with CTA” group (P=1.0) (Table 3). Fat necrosis also occurred at similar rates with the addition of CTA planning, six cases with CTA compared to seven cases without CTA (P=0.5). The percentage of abdominal bulge in the group with preoperative CTAs was only 6% compared to 13% (P=0.6). The average rate of blood transfusion received between the two groups were similar but lower unplanned intensive care unit admissions.
Table 3
| Complications | Without CTA (n=16) | With CTA (n=18) | P value |
|---|---|---|---|
| Recipient site, n [%] | |||
| Partial flap loss | 1 [6] | 2 [11] | 1.0 |
| Total flap loss | 0 | 0 | 1.0 |
| Mastectomy flap and nipple necrosis | 2 [13] | 3 [17] | 1.0 |
| Flap fat necrosis | 7 [44] | 6 [33] | 0.5 |
| Breast haematoma | 2 [13] | 0 [0] | 0.2 |
| Breast seroma | 0 | 0 | – |
| Breast infection | 2 [13] | 1 [6] | 0.6 |
| Donor site, n [%] | |||
| Abdominal haematoma | 0 | 0 | – |
| Abdominal seroma | 1 [7] | 2 [11] | 1.0 |
| Abdominal infection | 1 [6] | 2 [11] | 1.0 |
| Hernia | 2 [13] | 3 [17] | 1.0 |
| Abdominal bulge | 2 [13] | 1 [6] | 0.6 |
| Revision abdominal closure | 2 [13] | 2 [11] | 1.0 |
| General, n [%] | |||
| VTE/PE | 0 | 1 [6] | 1.0 |
| Blood transfusion received | 4 [25] | 6 [33] | 0.8 |
| Unplanned ICU admission | 2 [13] | 1 [6] | 0.59 |
P values calculated using t-test or χ2-test. CTA, computed tomographic angiogram; VTE, venous thromboembolism; PE, pulmonary embolism; ICU, intensive care unit.
Discussion
Preoperative CTA can provide a wealth of information to assist in surgical planning. It provides a map of the vascular supply of the abdominal wall—of most relevance the presence and pattern of the superior and inferior deep epigastric system. These scans are accurate in determining the location, course, patency and calibre of musculocutaneous perforators down to a diameter of 0.3 mm (12). CTA also assists in characterising soft tissue structures of the abdominal wall, such as hernias and scar tissue, and demonstrate their relationship and impact on vascular supply.
The role of preoperative CTA in free abdominal tissue flap breast reconstruction has been well established. It allows surgeons to select the appropriate patients, perforators and donor sites in the preoperative setting (13,14). CTA allows visualisation of the size and branching patterns of the deep inferior epigastric artery (DIEA) and its course through to abdominal wall. Information on the size of DIEA perforators assists the surgeon in preferencing the optimal hemiabdomen with the largest perforators located in the ideal position on the skin paddle. This preoperative knowledge has led to safer and more efficient dissection of the pedicle with reduced operative times (9,15,16). These factors have culminated in improved vascularity of the flaps raised and reduced ischaemia-related complications such as skin and fat necrosis (16,17).
The most significant benefit of CTA planning for pedicled TRAM flaps is to minimise donor site morbidity.
The aetiology of hernia and bulge occurring after abdominal flap harvest is multifactorial (18). One of the primary causes is from to laxity and attenuation in the anterior rectus sheath. Sacrifice and dissection of the rectus abdominis muscle also contributes to muscle atrophy and weakness in the abdominal wall. The map of vessels and perforators provided by the CTA provides the ability for maximal sparing of anterior rectus sheath and muscle in the pedicled TRAM, whilst maintaining flap vascularity. This sheath preservation improves abdominal wall integrity after repair and decreases the occurrence of abdominal bulge or hernia. A medial or lateral strip of rectus muscle may also be spared which preserves some function of the muscle and further improve abdominal integrity. This benefit was reflected in our results with less than half the number of abdominal bulges occurring in the CTA group.
In unilateral autologous breast reconstruction with pedicled TRAM flaps prior to the use of preoperative CTA, the senior author used both contralateral and ipsilateral TRAM flaps equally (56% and 44% respectively). However, this practice has changed after the adoption of preoperative CTA since late-2015, where flap choice was influenced by size and locations of the perforators exiting the rectus abdominis muscles in accordance with Poiseuille’s law (19). With the use of CTA for preoperative planning, the contralateral flap was used in 78% of cases.
In planning for pedicled TRAM flap, a CTA provides a multitude of information to ensure the optimal vascularity to the flap. Preoperative CTA can ensure the presence and measure the calibre of the SEA on which the pedicled TRAM is based and show the location and size of the perforators the SEA supplies. This is essential in patients with previous abdominal surgery where these vessels may be transected, in particular those with subcostal incisions after open cholecystectomy or splenectomy. The size, number and location of perforators allows the surgeon to predict the size of the flap and the extent of its vascularity into adjacent zones.
DIEA and similarly SEA branching patterns are characterised on CTA as type 0–4 based on number of branches. An anatomical study by Moon has shown that the number of SEA branches is proportional to the number of anastomoses between the superior and inferior epigastric systems in the periumbilical region (20). Anatomical symmetry of vascular patterns between hemiabdomens occurs in only 2% of cases, so measurement of SEA and perforator calibre and branching with CTA will allow the optimally vascularised hemiabdomen to be chosen preoperatively (20). This planning would be expected to decrease associated complications of skin and fat necrosis postoperatively.
In our cohort of 34 pedicled TRAM flaps there were no significant difference in outcomes between the two groups. It was postulated that there may be an associated reduced operative time and reduced complication rate in the “with CTA” group, however this was not reflected in our dataset with statistical significance. This was likely due to the small sample size of the cohort and retrospective nature of the study.
In clinical practise, the role for CTA imaging must be weighed against the risks and side-effects of this intervention. CTA does carry a financial cost, and this is not insignificant in health systems with finite resources. Similarly, access to CTA is not uniform and may limit other patients’ access. The scan itself requires exposure to ionising, and the use of contrast agents can be associated with nephrotoxicity and allergic reactions.
Conclusions
The pedicled TRAM flap is a well-established reliable and robust reconstructive option for both immediate or delayed autologous breast reconstruction. The use of CTA for perforator mapping, that is already well established for use in DIEP and free TRAM flaps, can be applied to the planning of pedicled TRAM flaps. This information provides the surgeon with a map of the anatomical features for optimal perforator selection and minimising rectus sheath harvest. Although CTA guidance was not shown to significantly reduce operating time or complication rates in this study, it provides the surgeon with greater confidence whilst undertaking a pedicled TRAM breast reconstruction. In particular, it allows maximal sparing of the abdominal wall components whilst maintaining flap vascularity. Further study and risk/benefit analysis may better highlight the role of CTA in pedicled TRAM flap planning.
Acknowledgments
The authors thank Vicky Tobin for her assistance in the statistical analysis for this paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-529/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-529/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-529/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-529/coif). WMR serves as an unpaid Associate Editor of Gland Surgery from March 2013 to February 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institutional ethics approval was obtained at both centres: Monash Health (registration No. RES-19-0000-740Q) and Peninsula Health (registration No. ERM # 86700), and all eligible patients were included and provided informed consent as required. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee BT, Agarwal JP, Ascherman JA, et al. Evidence-Based Clinical Practice Guideline: Autologous Breast Reconstruction with DIEP or Pedicled TRAM Abdominal Flaps. Plast Reconstr Surg 2017;140:651e-64e. [Crossref] [PubMed]
- Ireton JE, Kluft JA, Ascherman JA. Unilateral and Bilateral Breast Reconstruction with Pedicled TRAM Flaps: An Outcomes Analysis of 188 Consecutive Patients. Plast Reconstr Surg Glob Open 2013;1:1-7. [Crossref] [PubMed]
- Ascherman JA, Seruya M, Bartsich SA. Abdominal wall morbidity following unilateral and bilateral breast reconstruction with pedicled TRAM flaps: an outcomes analysis of 117 consecutive patients. Plast Reconstr Surg 2008;121:1-8. [Crossref] [PubMed]
- Chun YS, Sinha I, Turko A, et al. Outcomes and patient satisfaction following breast reconstruction with bilateral pedicled TRAM flaps in 105 consecutive patients. Plast Reconstr Surg 2010;125:1-9. [Crossref] [PubMed]
- Schwitzer JA, Miller HC, Pusic AL, et al. Satisfaction following Unilateral Breast Reconstruction: A Comparison of Pedicled TRAM and Free Abdominal Flaps. Plast Reconstr Surg Glob Open 2015;3:e482. [Crossref] [PubMed]
- Larson DL, Yousif NJ, Sinha RK, et al. A comparison of pedicled and free TRAM flaps for breast reconstruction in a single institution. Plast Reconstr Surg 1999;104:674-80. [Crossref] [PubMed]
- Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the deep inferior epigastric artery: a blinded, prospective cohort study. Plast Reconstr Surg 2008;122:1003-9. [Crossref] [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Grinsell D, et al. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery 2008;28:306-13. [Crossref] [PubMed]
- Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg 2010;63:1597-601. [Crossref] [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [Crossref] [PubMed]
- Piorkowski JR, DeRosier LC, Nickerson P, et al. Preoperative computed tomography angiogram to predict patients with favorable anatomy for superficial inferior epigastric artery flap breast reconstruction. Ann Plast Surg 2011;66:534-6. [Crossref] [PubMed]
- Masia J, Larrañaga J, Clavero JA, et al. The value of the multidetector row computed tomography for the preoperative planning of deep inferior epigastric artery perforator flap: our experience in 162 cases. Ann Plast Surg 2008;60:29-36. [Crossref] [PubMed]
- Tong WM, Dixon R, Ekis H, et al. The impact of preoperative CT angiography on breast reconstruction with abdominal perforator flaps. Ann Plast Surg 2012;68:525-30. [Crossref] [PubMed]
- Fitzgerald O'Connor E, Rozen WM, Chowdhry M, et al. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg 2016;5:93-8. [Crossref] [PubMed]
- Baumann DP, Lin HY, Chevray PM. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg 2010;125:1335-41. [Crossref] [PubMed]
- Nahabedian MY, Dooley W, Singh N, et al. Contour abnormalities of the abdomen after breast reconstruction with abdominal flaps: the role of muscle preservation. Plast Reconstr Surg 2002;109:91-101. [Crossref] [PubMed]
- Blondeel PN, Morris SF, Neligan P, et al. Perforator Flaps: Anatomy, Technique, & Clinical Applications. 2nd edition. New York: Thieme Medical Publishers Inc.; 2013.
- Moon HK, Taylor GI. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg 1988;82:815-32. [Crossref] [PubMed]


