
Paratesticular fibrous pseudotumor with histological features of IgG4-related disease: two case reports and review of the literature
Highlight box
Key findings
• Paratesticular fibrous pseudotumor is a rare pathological entity, counting only 6% of all paratesticular masses.
What is known and what is new?
• Based on morphological similarities to other sclerosing fibro-inflammatory disorders, characterized by IgG4-expressing plasma cells, it might belong to the IgG4-related disease family.
• A history of trauma, infections, surgery and inflammatory events has been suggested to be associated with development of this condition.
What is the implication, and what should change now?
• Paratesticular pseudotumor is curable with conservative approach.
Introduction
Despite representing the second commonest paratesticular lesion after adenomatoid tumor, paratesticular fibrous pseudotumor (PFP) is a rare pathological entity, counting only 6% of all paratesticular masses. Its incidence is extremely rare with only 200 cases reported in literature. It mainly affects young males with a peak incidence between the ages of 20 and 40 years (1).
First described by Sir Astley Cooper in 1830 as a fibromatous lesion and by Balloch in 1904 with the term “fibromata” (2), this entity has been known with many terms: “chronic proliferative periorchitis”, “nonspecific peritesticular fibrosis”, “proliferative funiculitis”, “fibromatous periorchitis”.
Actually and widely adopted nomenclature of PFP was coined by Mostofi and Price who in 1973 described a condition characterized by solitary or multiple intrascrotal nodules, microscopically composed of dense, almost acellular, hyalinized collagen with variable sparse inflammatory infiltrate, supporting its reactive and benign rather than neoplastic origin (3).
PFP can arise from testicular tunica layers, vaginalis and albuginea, along the spermatic cord or the epididymis.
Although the etiology is still unknown, a history of trauma, infections, surgery and inflammatory events has been suggested to be associated with development of this condition.
Based on morphological similarities to other sclerosing fibro-inflammatory disorders, characterized by IgG4-expressing plasma cells, Bösmüller et al. in 2011 (1) postulated that PFP might belong to the IgG4-related disease (IgG4-RD) family, which includes heterogeneous entity like retroperitoneal fibrosis, sclerosing pancreatitis, sclerosing cholangitis and Riedel’s thyroiditis.
Due to its rarity, misdiagnosis with other entities and heterogeneity of the terminology, only few cases have been reported in the literature supporting the correlation between PFP and IgG4-RD.
This entity, due to its benign nature, is not listed in the Classification of Tumours of the Urinary System and Male Genital Organs.
Nevertheless, management of PFP could be extremely challenging: due to the lack of typical clinical signs and the non-specific radiological characteristics, misapprehension does occur in the majority of cases, mainly because these intrascrotal mass may mimic testicular neoplasm, therefore leading to radical orchidectomy rather than a desirable testis-sparing surgery (4-7).
Herein, we report two cases of PFP of the spermatic cord with histological features suggestive for IgG4-RD as well as a review of the literature. We present the following article in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-290/rc).
Case presentation
Both of our cases presented as slow growing lesions of the spermatic cord.
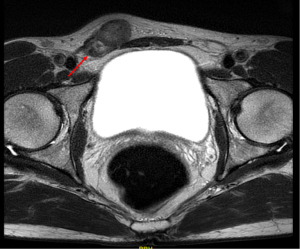
The first case was a 45-year-old man, with no significant medical or surgical history who presented at our institution with an asymptomatic, slowly growing, palpable right inguinal mass. Physical examination revealed a hard consistency, multinodular mobile mass along the right spermatic cord near the upper pole of the testis that was apparently not involved. Scrotal ultrasound (US) revealed normal testicles and a paratesticular heterogeneous hyperechoic lesion of 3.5 cm in diameter, along the right spermatic cord cranial to the testicle without tunica albuginea involvement. A magnetic resonance imaging (MRI) was then performed revealing a diffuse-proliferated nodular mass with iso- and low-intensity signal in T1- and T2-weighted imaging and low-contrast agent uptake (Figure 1). Laboratory investigation revealed negative lactate dehydrogenase (LDH), alpha-fetoprotein and beta-human chorionic gonadotropin (beta-hCG). Patient underwent an inguinal surgical exploration and intraoperative frozen section lay for a benign tumor. A testicular-sparing surgery with enucleation of the mass was then performed and concomitant orchiopexy.
The second case was a 28-year-old man, with no significant medical or surgical history, who presented with an asymptomatic, slowly growing, palpable left intra-scrotal mass. Physical examination revealed a mobile mass along the left spermatic cord without involvement of the testis. Scrotal US revealed normal testicles and a paratesticular heterogeneous hyperechoic lesion of 2 cm in diameter, along the left spermatic cord cranial to the testicle.
Laboratory investigation revealed negative LDH, alpha-fetoprotein and beta-hCG. Also, in this case patient underwent an inguinal surgical exploration, intraoperative frozen section lay for a benign tumor. A testicular-sparing surgery with enucleation of the mass was then performed.
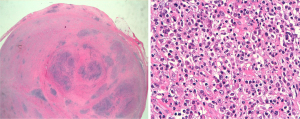
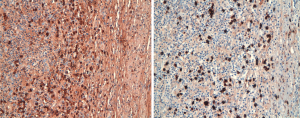
In both cases, masses presented with similar histo-morphological features. Grossly, both of the masses presented as well circumscribed, solid nodules with white-yellowish fasciculate cut surface. After fixation and paraffin embedding, routine hematoxylin-eosin (H&E) showed that the lesions were composed of paucicellular mesenchymal proliferation of myofibroblastic spindle cells with some areas showing storiform pattern, embedded in a dense fibrosclerotic tissue (Figure 2). A variable amount of inflammatory infiltrate composed of nodular aggregates of lymphocytes, numerous plasma cells and scattered eosinophils was also present. There was no evidence of phlebitis. The spindle cells showed immunohistochemical positivity for caldesmon, while they were negative for smooth muscle actin, S100, calretinin, desmin, ALK-1 and beta-catenin. The lymphoid infiltrate was composed of a mixture of small B (CD20 positive) and T (CD3 positive) elements. The plasma cells were not light chain restricted, and showed positivity for IgG and IgG4, with IgG4 number >40/high power field (HPF) and proportion of IgG4/IgG >40% in some areas (Figure 3). Condo red stain was negative. The final diagnosis was of PFP with histological aspects suggestive for IgG4 related disease.
At 2-year follow-up, no evidence of local or distant relapse nor testicular disorder was observed in both patients. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from both patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PFP with histological features of IgG4-RD are very rare conditions. A non-systematic review of the literature was performed in December 2020, using the PubMed/MEDLINE database applying the following terms and combinations: “paratesticular fibrous pseudotumor”, “IgG4-related disease”, “inflammatory pseudotumor”. Original articles, reviews, case reports and case series were selected. Only English-language papers were included. Due to the rarity of spontaneous regression, no time restriction was applied. Despite the peak incidence of PFP is the third-forth decade of life, as for our patients, some authors reported older mean age at presentation (8,9). Nevertheless, due to slow growth rate, the real age onset is unknown, with patients seeking medical advice only after several months or years from the appearance of scrotal masses, and this could partly explain the extreme rarity of this entity in pre-puberty age.
As reported in the literature, our patients were both asymptomatic with scrotal swelling as the only awareness sign of PFP. In both our patients, PFP involved the spermatic cord while it is usually reported to arise from tunica vaginalis (76% of cases), tunica albuginea (14%) and epididymis (10%) (3). Although great variability in size has been described, from 0.5 to >8 cm (8) the size of PFP in both our cases were in line with the mean size reported literature.
The preoperative diagnosis of PFP is challenging due to non-specific findings on imaging. On US, PFP may present as single or multiple, either hyperechoic or hypoechoic, lesions depending on the degree of calcification, hyalinization and inflammatory granulation tissue (10). In our patients US aspect of PFPs was of non-specific heterogeneous hyperechoic lesions. No data are available in literature regarding the added value of a contrast-enhanced US.
Although report with MRI is limited in literature, some authors have reported a specific appearance with PFP that exhibits intermediate-to-low signal intensity on T1- and T2-weighted images with little or absent contrast enhancement. Our experience showed similar observation suggesting the added value of scrotal MRI in preoperative diagnosis of PFP, nevertheless radiologist expertise is essential (11). Laboratory and image staging with tumor markers and thoraco-abdominal computed tomography (CT) scan are mandatory in the differential diagnosis with testicular and paratesticular malignancies.
Considering its benign behavior, a testis-sparing surgery is desirable. Nevertheless, due to the challenging in clinical and imaging diagnosis of PFP and its trend to mimic testicular neoplasm, radical orchiectomy has been usually reported as primary treatment (4-7).
A recent study highlighted the added value of frozen section assessment in testicular and paratesticular lesions in avoiding unnecessary radical orchiectomy up to 84% for benign diagnosis (12). In both our patients, frozen section assessments were consistent for benign lesions therefore a testis-sparing surgery was performed.
Nevertheless, it must be taken into consideration that despite the undoubted advantage and ease in performing a trans-scrotal approach, due to the intrinsic anatomical risk of metastatic seeding in case of malignancies, an inguinal approach is desirable.
Bösmüller et al. 10 years ago hypothesize the correlation between PFP and IgG4-RDs based on morphological evaluation and quantitative assessment of IgG4 plasma cells (1). Later other authors confirmed these findings (13,14). Furthermore, the onset of PFP in the contest of a systemic IgG4-RD and the regression of the paratesticular mass after corticosteroid therapy documented by others supported the intuition that PFP could be a local manifestation of a systemic disease (9,14,15).
IgG4-RD is an immune-mediated fibro-inflammatory condition initially described in the pancreas as a specific type of autoimmune pancreatitis, later recognized as capable of affecting multiple organs (16). The physiopathology of this entity is still, not clear as well as whether the role of increased IgG4 is primary or secondary. While the clinical presentation is variable and related to the localization of the disease, the morphological features are similar, so that histology is crucial for the correct diagnosis. The major histopathological features associated with IgG4-RD are the presence of a lymphoplasmacytic infiltrate, fibrosis, arranged at least focally in a storiform pattern, phlebitis with or without obliteration of lumen, and eosinophils. IgG4 immunostaining is required for a quantitative assessment of the absolute count of IgG4-positive plasma cells and for the IgG4/IgG ratio. The presence of >50 IgG4 plasma cells per high powder field is considered highly specific, although it is organ related; an IgG4/IgG plasma cells ratio of 40% is suggested as a powerful cut-off value in any organ (16). Elevated serum IgG4 levels are often but not always present, although they are not specific.
In our case, most of the morphological criteria for IgG4-RD were satisfied as well as immunohistochemical assessment of IgG4-positive plasma cells that were >50/HPF and with a IgG4/IgG ratio >40%. Negativity for smooth muscle actin, desmin, S100, calretinin, ALK-1 and beta-catenin helps to rule out a diagnosis of leiomyoma, benign peripheral nerve sheath tumors, fibromatosis and inflammatory myofibroblastic tumor (17).
In both our patients, serum IgG4 levels were not evaluated preoperatively since PFP was initially not suspected. Postoperative serum IgG4 levels were normal, nevertheless it is not known whether it is due to PFP excision or not.
Unfortunately, there was no clinical correlation to determine if both patients had other manifestation of IgG4-RD.
Conclusions
Despite the increasing reports and series about PFP and IgG4 related disease, the etiopathogenesis of this phenomenon is still not clear. The morphological and immunohistochemical characteristic in our cases support the theory of PFP being part of IgG4-RD family, thus the knowledge of this entity is crucial for the optimal management. If clinical and US diagnosis are inconclusive, scrotal MRI could be performed to aid the preoperative diagnosis. Testicular-sparing surgery, combined with frozen section assessment should be the optimal management for PFP. Further research is needed in order to fully characterized this phenomenon in all its biology, etiopathogenesis and clinical management.
Acknowledgments
Funding: This research received “Ricerca Corrente” funding from the Italian Ministry of Health to cover publication costs.
Footnote
Reporting Checklist: The authors have completed CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-290/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-290/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-290/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from both patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bösmüller H, von Weyhern CH, Adam P, et al. Paratesticular fibrous pseudotumor--an IgG4-related disorder? Virchows Arch 2011;458:109-13. [Crossref] [PubMed]
- Balloch EA. IX. Fibromata of the Tunica Vaginalis. Ann Surg 1904;39:396-402. [Crossref] [PubMed]
- Mostofi FK, Price EB. Tumors of the male genital system. In: Firminger HI, editor. Atlas of tumor pathology. 2nd series, fascicle 8. Washington DC: Armed Forces institute of Pathology; 1973:151-4.
- Seethala RR, Tirkes AT, Weinstein S, et al. Diffuse fibrous pseudotumor of the testicular tunics associated with an inflamed hydrocele. Arch Pathol Lab Med 2003;127:742-4. [Crossref] [PubMed]
- Tarhan H, Divrik RT, Akarken I, et al. Benign intrascrotal lesion: fibrous pseudotumor of testis. Arch Ital Urol Androl 2011;83:105-7.
- Ugras S, Yesil C. Fibrous pseudotumors of tunica albuginea, tunica vaginalis and epididymis: report of two cases. Cancer Epidemiol 2009;33:69-71. [Crossref] [PubMed]
- Başal Ş, Malkoç E, Aydur E, et al. Fibrous pseudotumors of the testis: The balance between sparing the testis and preoperative diagnostic difficulty. Turk J Urol 2014;40:125-9. [Crossref] [PubMed]
- Miyamoto H, Montgomery EA, Epstein JI. Paratesticular fibrous pseudotumor: a morphologic and immunohistochemical study of 13 cases. Am J Surg Pathol 2010;34:569-74. [Crossref] [PubMed]
- Dieckmann KP, Struss WJ, Frey U, et al. Paratesticular fibrous pseudotumor in young males presenting with histological features of IgG4-related disease: two case reports. J Med Case Rep 2013;7:225. [Crossref] [PubMed]
- Bulakci M, Tefik T, Kartal MG, et al. Imaging Appearances of Paratesticular Fibrous Pseudotumor. Pol J Radiol 2016;81:10-4. [Crossref] [PubMed]
- Cassidy FH, Ishioka KM, McMahon CJ, et al. MR imaging of scrotal tumors and pseudotumors. Radiographics 2010;30:665-83. [Crossref] [PubMed]
- Subik MK, Gordetsky J, Yao JL, et al. Frozen section assessment in testicular and paratesticular lesions suspicious for malignancy: its role in preventing unnecessary orchiectomy. Hum Pathol 2012;43:1514-9. [Crossref] [PubMed]
- Kodama H, Hatakeyama S, Matsumoto T, et al. A Case of Fibrous Pseudotumor in the Scrotum: Challenge for Diagnosis and Testicular Preservation. Case Rep Urol 2018;2018:6904827. [Crossref] [PubMed]
- ChangChien YC. Paratesticular Fibrous Pseudotumor: A New Entity of IgG4-Related Disease? Ann Clin Lab Sci 2018;48:381-5.
- Kim KH, Sung DJ, Han NY, et al. Immunoglobulin G4-related paratesticular fibrous pseudotumor and retroperitoneal fibrosis: a case report. Urol Int 2015;94:369-72. [Crossref] [PubMed]
- Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 2003;38:982-4. [Crossref] [PubMed]
- Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181-92. [Crossref] [PubMed]


