
Assessing the preventive effect of immediate lymphatic reconstruction on the upper extremity lymphedema
Highlight box
Key findings
• An immediate lymphatic reconstruction (ILR) combining axillary reverse lymphatic mapping and lymphovenous anastomosis showed a promising result for prevention of lymphedema.
What is known and what is new?
• ILR has been gradually in the spotlight as a novel surgical technique to prevent lymphedema, but most clinical studies were highly biased and did not show statistical evidence, because they lacked control groups.
• We compared the incidence of postoperative lymphedema between the ILR treatment group (n=30) and the no-try or failure group (n=183) during the same period.
• In multivariate Cox’s proportional hazard regression analysis, ILR success showed a borderline significant decrease in risk of lymphedema (HR =0.174; 95% CI: 0.022–1.374; P=0.097).
What is the implication, and what should change now?
• ILR may serve as an effective option for prevention, especially in high-risk patients who are planning to undergo axillary lymph node dissection and radiotherapy.
Introduction
Lymphedema is a debilitating disease that causes the lymphatic vessel injury, fat hypertrophy, tissue fibrosis, and ultimately skin ulceration and infections (1). In the upper extremity, most secondary lymphedema occurs after axillary lymph node dissection (ALND) following breast cancer surgery, with an estimated toll of 20 to 45 percent prevalence (2). Despite the development of microsurgical techniques such as lymphovenous anastomosis (LVA), vascularized lymph node transfer (VLNT) as well as conservative management, including manual lymphatic drainage, pneumatic pumps, and compression garments, there is still no cure for lymphedema.
In recent years, an immediate lymphatic reconstruction (ILR) has been gradually in the spotlight as a novel surgical technique to prevent lymphedema (3-8). With an advent of axillary reverse lymphatic mapping (ARLM), surgeons can identify the lymphatic drainage pathway from an upper extremity. An ILR technique combining ARLM and LVA has shown encouraging results in reducing the risk of developing breast cancer-related lymphedema (BCRL) about a third (7-11). This preventive approach, reducing the likelihood before the disease occurs, is suggesting a new paradigm shift in the treatment of lymphedema.
However, most clinical studies of ILR were highly biased and did not show sufficient statistical evidence, because they lacked control groups (7,10). Even studies with control groups, did not secure standardization for statistical analysis, because they set up a control group consisting of patients for whom the surgeon could not perform ILR. Last, most of the studies used ambiguous diagnostic criteria for “lymphedema” and did not adjust many confounding variables.
In this study, we present the outcomes for our patients who underwent an ILR procedure to prevent BCRL. We will compare the incidence of postoperative lymphedema between the ILR success group and no-try or failure group during the same period. Furthermore, we will assess the preventive effects of ILR for the risk of BCRL by analyzing the effects of different variables. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-554/rc).
Methods
Patients
Medical records including detailed operation notes, follow-up records, and photographs were collected and analyzed. The patients (I) whose medical records were unclear or without clinical photos and (II) who had less than 6 months of follow-up were excluded. Also, patients (III) who had recurrent breast cancer, (IV) who underwent prophylactic mastectomy, or (V) who had already had symptoms of lymphedema before mastectomy were excluded to achieve standardization. As a result, a total of 213 patients who underwent mastectomy for node-positive unilateral breast cancer between November 1, 2019 and February 28, 2021 were included regardless of whether they had undergone ILR or not.
Surgical technique
In our center, ILR is indicated for patients who are scheduled for ALND and agreed to the operation. Three oncologic surgeons decided on the type of axillary nodal intervention intraoperatively. Following axillary surgery, the first author performed all attempted ILRs at the time of nodal extirpation.
Before the initiation of the axillary procedure, ARLM was performed in an area 6 cm distal to the axilla as previously described by Thompson (12). A total of 3 cc of isosulfan blue dye was injected into 5 to 6 spots into the subdermal plane. Then, massage was performed from the injection site so that dye could flow well to the axilla.
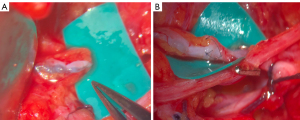
Following completion of the ALND, arm lymphatics and an axillary vein branch were investigated under an operating microscope. Blue lymphatics or blue nodes were identified in most cases, but if not, exploration was performed within anticipated course of arm lymphatics. Afferent lymphatic vessels, which were too small in diameter or too far away for anastomosis, were clipped. After identifying a branch of an axillary vein of the appropriate size in the vicinity, ILR was performed with 10-0 nylon sutures (Ethicon, Somerville, NJ, USA) with an end-to-end fashion. As described by Boccardo et al., after introducing the lymphatic vessel into the vein with the first U stitch, perilymphatic tissues and venous walls were anastomosed with a interrupted suture (Figure 1) (9). If the vein was larger than the lymphatic vessel, end-to-side anastomosis was performed. We performed one anastomosis per each patient, then patency of the anastomosis was confirmed under the microscope and indocyanine green (ICG) lymphography.
Surveillance
In our center, patients treated for breast cancer were regularly followed up every 6 months with mammography and ultrasonography for the 5 years and annually thereafter, depending on the primary pathological condition. Furthermore, patients who received ILR were additionally followed up every three months up to 2 years after surgery. At each visit, limb circumference was measured at the following six points: the superior margin of the upper arm, mid upper arm, the superior and inferior margins of the elbow, mid forearm, and wrist. Also, patients were closely monitored for any signs or symptoms consistent with lymphedema (e.g., swelling, heaviness). If there were any suspected symptoms, patients were evaluated through consultation with a rehabilitation physician, and if necessary, additional imaging work-up such as lymphoscintigraphy was done.
Lymphedema is defined at our institution as (I) having symptoms consistent with lymphedema and (II) being diagnosed by a certified rehabilitation physician. If there was a positive finding in the imaging modality or limb circumference, patients were also considered to have lymphedema.
Study design
The diagnosis of lymphedema was the primary end point of this study. If there were no events, the observation was censored at the last follow-up visit. To assess the effect of ILR on the risk of upper extremity lymphedema, we compared outcomes between the ILR success group (n=26) and no-try or failure group (n=187) during the same period.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Korea University Anam Hospital (protocol number 2021AN0524). Individual consent for this retrospective analysis was waived.
Statistical analyses1
In patient demographic analysis, categorical variables are expressed as counts (percentages) with Chi-squared tests or Fisher’s exact tests. Continuous variables are summarized by means (with standard deviations) or medians (interquartile ranges) with two independent t-tests or Mann-Whitney tests depending on whether normality was satisfied.
Univariate and multivariable Cox’s proportional hazards regression models were used to analyze the effects of the ILR success compared to the control group for lymphedema occurrence after adjusting for clinicopathologic variables. The variables such as age, body mass index (BMI), comorbidity (hypertension, diabetes), smoking, tumor grade, type of mastectomy, type of ALND, reconstructive surgery, chemotherapy, radiotherapy, hormonal therapy and ILR success were included in the multivariable Cox’s proportional hazard regression model. The final model with some important factors was obtained by stepwise variable selection method. The proportional hazards assumption was checked using a Supremum test and graphical diagnostics based on the scaled Schoenfeld residuals. For all analyses, a value of P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA) and SAS, version 9.4. (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics
We analyzed a total of 213 patients including 30 patients who had undergone an ILR attempt and 183 who had received axillary nodal intervention without ILR attempts during the same period. The mean follow-up period was 14 months (range, 6–21 months). There was no significant difference between the groups in age, BMI, comorbidity (hypertension and diabetes), smoking history, tumor grade, or hormonal therapy (Table 1). However, the rates of patients who had undergone modified radical mastectomy in the ILR group (66.7% vs. 44.3%, P<0.001) tended to be higher, and thus the rate of having chemotherapy (93.3% vs. 56.3%, P<0.001) and radiotherapy (60.0% vs. 22.4%, P<0.001) was significantly higher than that of the control group. In the ILR group, most patients had undergone ALND (93.3%) rather than sentinel node biopsy.
Table 1
| Variables | Patient who underwent ILR attempts (%) | Control (%) | P value |
|---|---|---|---|
| No. of patients | 30 (14.1) | 183 (85.9) | |
| Age, mean ± SD, years | 52.27±10.0 | 52.10±12.0 | 0.94 |
| BMI, mean ± SD, kg/m2 | 24.57±3.06 | 23.44±3.69 | 0.11 |
| Hypertension | 6 (20.0) | 31 (16.9) | 0.68† |
| Diabetes mellitus | 0 (0.0) | 17 (9.3) | 0.14‡ |
| Smoking history | 1 (3.3) | 2 (1.1) | 0.37‡ |
| Tumor grade | 0.10‡ | ||
| 0 | 2 (6.7) | 9 (4.9) | |
| I | 2 (6.7) | 31 (16.9) | |
| II | 13 (43.3) | 98 (53.6) | |
| III | 12 (40.0) | 44 (24.0) | |
| Uncategorized | 1 (3.3) | 1 (0.5) | |
| Mastectomy type | <0.001†* | ||
| MRM | 20 (66.7) | 81 (44.3) | |
| NSM | 1 (3.3) | 36 (19.7) | |
| SSM | 3 (10.0) | 63 (34.4) | |
| BCS | 6 (20.0) | 3 (1.6) | |
| Lymph node dissection | <0.001†* | ||
| ALND | 28 (93.3) | 57 (31.1) | |
| SLND | 2 (6.7) | 126 (68.9) | |
| Reconstruction | 8 (26.7) | 105 (57.4) | 0.001†* |
| Autologous flap | 3 (10.0) | 35 (19.1) | |
| DIEP | 3 (10.0) | 28 (15.3) | |
| LD | 0 | 7 (3.8) | |
| Implant-based | 5 (16.7) | 79 (43.2) | |
| Oncoplastic surgery | 0 | 1 (0.5) | |
| Chemotherapy | 28 (93.3) | 103 (56.3) | <0.001†* |
| Neoadjuvant | 20 (66.7) | 57 (31.1) | |
| Adjuvant | 16 (53.3) | 55 (30.1) | |
| Radiotherapy | 18 (60.0) | 41 (22.4) | <0.001†* |
| Hormonal therapy | 9 (30.0) | 79 (43.2) | 0.18† |
Patients underwent implant-enhanced LD flap were classified to LD flap. Above P value was calculated by chi-square test or Fischer exact test or t-test or Mann-Whitney test for the difference between “immediate lymphatic reconstruction” group and control group. †, Chi-square analysis; ‡, Fisher’s exact test; *, statistical significance. ILR, immediate lymphatic reconstruction; SD, standard deviation; BMI, body mass index; MRM, modified radical mastectomy; NSM, nipple sparing mastectomy; SSM, skin sparing mastectomy; BCS, breast conserving surgery; ALND, axillary lymph node dissection; SLND, sentinel lymph node dissection; DIEP, deep inferior epigastric perforator flap; LD, latissimus dorsi.
Surveillance and postoperative outcomes
Table 2 summarizes the postoperative results of ILR. Of the 30 patients who were attempted, ILRs were successfully performed in 26 patients (86.7%). For the anastomosis method, end-to-end fashion was the most commonly performed (n=18, 69.2%). In the ILR group, one patient (3.8%) was confirmed to have upper extremity lymphedema 20 months after ALND. In the control group, on the other hand, 14 out of 183 patients (7.7%) were diagnosed postoperative lymphedema.
Table 2
| Variables | Patient who underwent ILR attempts (%) | Control (%) | P value |
|---|---|---|---|
| No. of patients | 30 | 183 | |
| ILR success | 26 (86.7) | N/A | |
| End-to-end anastomosis | 18 (69.2) | ||
| End-to-side anastomosis | 8 (30.8) | ||
| Postoperative lymphedema | 1 (3.8) | 14 (7.7) | 0.70† |
†, Fisher’s exact test. ILR, immediate lymphatic reconstruction.
Risk factors for postoperative lymphedema
To assess the risk of postoperative lymphedema, we used univariate and multivariate Cox’s hazard regression models for statistical analysis including the time variable. In the univariate model, ALND [HR =7.375; 95% confidence interval (CI): 2.074–26.223], chemotherapy (HR =4.492; 95% CI: 1.013–19.917), and radiotherapy (HR =4.589; 95% CI: 1.622–12.990) showed an increased hazard ratio with statistical significance (Table 3).
Table 3
| Independent variable | OR | 95% confidence interval | P value |
|---|---|---|---|
| Age of >60 years | 0.852 | 0.271–2.680 | 0.785 |
| Obesity (BMI >25 kg/m2) | 1.586 | 0.447–5.624 | 0.475 |
| Hypertension† | 2.222 | 0.757–6.536 | 0.146 |
| Diabetes mellitus | 1.341 | 0.176–10.228 | 0.777 |
| Smoking | 6.173 | 0.794–47.619 | 0.082 |
| Tumor grade | 0.871 | 0.489–1.550 | 0.639 |
| Type of mastectomy | 0.858 | 0.503–1.464 | 0.575 |
| Axillary lymph node dissection | 7.375 | 2.074–26.223 | 0.002* |
| Reconstructive surgery | 1.772 | 0.631–4.979 | 0.278 |
| Chemotherapy | 4.492 | 1.013–19.917 | 0.048* |
| Radiotherapy | 4.589 | 1.622–12.990 | 0.004* |
| Hormonal therapy | 1.184 | 0.419–3.345 | 0.749 |
| ILR success | 0.577 | 0.076–4.392 | 0.596 |
*, statistical significance; †, borderline significance. OR, odds ratio; BMI, body mass index; ILR, immediate lymphatic reconstruction.
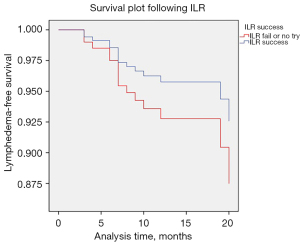
Table 4 summarizes the selected variables that showed statistical significance using stepwise selection. Of the above-mentioned variables, only ALND (HR =6.582; 95% CI: 1.654–26.198) was independently associated with postoperative lymphedema. ILR success showed a borderline significant decrease in risk of lymphedema (HR =0.174; 95% CI: 0.022–1.374; P=0.097). Figure 2 shows a survival plot by ILR success obtained after adjusting other variable.
Table 4
| Variables | Hazard ratio | P value | Hazard ratio (95% CI) |
|---|---|---|---|
| Hypertension (+) vs. hypertension (−) | 2.427 | 0.108 | 0.823 to 7.143 |
| ALND vs. SLND | 6.582 | 0.007* | 1.654 to 26.198 |
| Radiotherapy (+) vs. radiotherapy (−) | 2.500 | 0.112 | 0.808 to 7.752 |
| ILR success (+) vs. ILR fail or not attempted | 0.174 | 0.097† | 0.022 to 1.374 |
We used multivariable Cox’s hazard survival model for statistical analysis. Variables including age, BMI, comorbidity (hypertension, diabetes), smoking, tumor grade, mastectomy type, lymph node dissection type, reconstructive type, chemotherapy, radiotherapy, hormonal therapy, and ILR success were fitted to this model, and stepwise selection was performed. *, statistical significance; †, borderline significance. CI, confidence interval; ALND, axillary lymph node dissection; SLND, sentinel lymph node dissection; ILR, immediate lymphatic reconstruction; BMI, body mass index.
Discussion
Secondary lymphedema in upper extremities is a common complication of breast cancer treatment. It occurs in 25% of patients who underwent surgery, of which 75% are known to develop symptoms within a year (2). The probability of developing lymphedema increases not only because of axillary dissection, but also subsequent radiation therapy (13). In addition, it can arise at any time after operation, and since it is still difficult to cure, cautious observation and sufficient follow-up periods are required for both physician and patient.
The concept of an ILR was introduced first by Boccardo et al. in 2009 under the name of “Lymphatic Microsurgical Preventive Healing Approach (LYMPHA)”, which consisted of LVA between arm lymphatics and a collateral branch of an axillary vein following an axillary operation (2). In their follow-up study in 2014, encouraging results were announced, in that only 4.05% or patients who underwent ILR had lymphedema (9). Since then, many institutions have started to perform this procedure and tried to prove the preventive effect. However, there are not many relevant studies yet, and even they rarely compare results with those for a control group; so statistical analysis is still at a rudimentary level.
So far, there have been two studies with a control group about preventive ILR on an upper extremity. First, Boccardo et al. reported the outcome for 46 patients who had undergone LYMPHA for the prevention of arm lymphedema (3). In their study, lymphedema was diagnosed in one patient in the treatment group, while 7 occurred in the control group. Although their study was valuable as a pioneering result, there is no explanation for blinding or randomization. Also, the diagnosis of lymphedema was determined by volume measurement using formulae based on the circumferential measurement, there may have been selection bias (14). Second, Feldman et al. reported that 24 patients who had undergone successful LYMPHA showed a lower incidence of lymphedema than the control group. (12.5% vs. 50%) (4). However, their study followed patients for a somewhat short period (mean follow-up time was 6 months) and had a problem with selection bias, because the control group was limited to patients for whom preventive LYMPHA was not successful (10).
To the best of our knowledge, this article is the first clinical comparative study reporting the possibility of ILR for reducing the rate of postoperative lymphedema based on statistical analysis. In our cohort, there was one patient confirmed to have upper extremity lymphedema 20 months after axillary ILR. She was a textile worker, and there was a difference in circumference diameter from 1 to 2 cm depending on the location of each measurement site above the elbow. In overall incidence, our institutional lymphedema rate of 3.8 percent was similar to that of seminal studies of Boccardo et al. (4.05%) or Johnson et al. (3.1%) (9,15). One notable point is that the rate of postoperative lymphedema in the control group was 7.7% (14 out of 183 patients), which was significantly lower than the reported value, 14.1% to 29.4% in recent studies (16-20). Since our study included a significant percentage of patients with a follow-up period of less than one year, the longer the follow-up period, the higher the rate is expected.
In our study, out of 30 patients, four were unable to receive ILR successfully (86.7% success rate). There are several reasons for failure; the most difficult thing is that ARLM is not always successful. Prior studies evaluating ARLM have demonstrated success rates with a range of 61–93% in identifying lymphatic channels from an upper extremity (12,21,22). Similarly, our study showed an approximate 70% success rate in identifying lymphatic channels after ARLM. In the case of failure in visualizing, we should have performed an exploration under a microscope, which contributed to an increase of operating time. Among four patients, three had no suitable lymphatics identified and one had no suitable vein for anastomosis.
In assessing the preventive effect of ILR, there is an important issue about defining transient versus ongoing lymphedema. In several studies published so far, a separate category called “transient lymphedema” was created and classified (4,15,23). Generally, if a patient’s lymphedema was diagnosed and treated within 6 months of final oncologic treatment, it was defined as transient. This is a very important argument, because one might conclude that there is no therapeutic effect of ILR, depending on whether patients with temporary symptoms are included as ongoing lymphedema. For instance, Feldman et al. reported that 8.3% of their patients showed ongoing lymphedema, but the rate increased to 12.5% after including transient lymphedema (4). Among our patients, there was also one patient who complained of a temporary feeling of heaviness immediately after surgery. But there was no change in circumference, and symptoms are completely disappeared within two months.
In our opinion, it does not seem appropriate to classify “transient lymphedema” simply based on the patient’s feeling (i.e., heaviness, numbness) without positive findings in circumference or imaging modalities. Therefore, in this study, we have defined the lymphedema as (I) having a positive finding in the imaging modality or limb circumference, and (II) being diagnosis clinically by a certified rehabilitation physician. Since this ambiguity results from the characteristics of a disease entity, it will be important to control various variables associated with temporary symptoms and use appropriate statistical methods to analyze the preventive effect of ILR.
In our study, patients who had undergone ALND (HR =6.582; 95% CI: 1.654–26.198; P<0.05) showed a higher hazard ratio with statistical significance by a multivariable Cox’s hazard regression model. ALND is a well-known risk factor for lymphedema, which has an almost four times higher incidence rate than does sentinel lymph node biopsy (24,25). Among our 15 patients who were diagnosed with lymphedema, 13 (86.7%) received an ALND.
In this study, hypertension (HR 2.427; 95% CI: 0.823–7.143) and radiotherapy (HR 2.5; 95% CI: 0.808–7.752) also showed an increased hazard ratio for developing lymphedema. In the literature review, hypertension and radiotherapy have been consistently reported as precipitating factors for lymphedema. Rockson et al. reported that the presence of hypertension in 51 of 130 patients seemed to predispose the development of lymphedema (P<0.005) (26). Several studies also reported that axillary radiotherapy increase the risk of lymphedema from 1.91- to 4.285-fold (19,27,28).
Compared to previous studies, we newly investigated the possible risk reduction according to ILR success for developing lymphedema. Although it does not show statistical significance (P=0.097), our outcomes show the same trend as in many existing studies demonstrating the preventive effect of ILR. Although 0.05 of P value is generally taken as the cut-off value to represent a significant effect, it is also arbitrary (29). Borderline significance of this study means that the treatment effect is more likely to lie around the point estimate from the trial, rather than at the ends of the CI (30). Since this novel technique is still in the early stage, we reasonably anticipate that it will prove to have an advantage in reducing the rate of postoperative lymphedema once enough patients are obtained.
In another aspect, a recent report about delayed distally based prophylactic ILR raised our interest. Chen et al. pointed out the theoretical risk of conventional axillary ILR, such as oncological safety, proximal lymph-vein pressure imbalance, and questionable long-term patency (31). Since it is difficult to decide whether to perform ALND preoperatively, we also began the delayed ILR procedure under local anesthesia in some cases last year. So far, a total of 23 patients have undergone delayed ILR in our center, and there have been no cases of postoperative lymphedema. Since it has not yet been a year since the delayed procedures began, a longer follow-up period more than 2 years is required. Anyway, their argument is noteworthy in that it can solve the problem of the imbalance of lymph-vein pressure gradient between large vessels and small lymphatics. Moreover, it can be considered as an alternative if an unexpected ALND is performed.
There were several limitations in our study. First, our short-term follow up of less than two years and low sample size are not sufficient to clearly demonstrate a preventive effect against lymphedema. In the literature, most patients seem to present symptoms within the first two years (24,25). Therefore, a larger series with longer follow-up is needed to confirm our findings. If possible, it would be better to organize a multicenter-based randomized clinical trial. It would also be more appropriate to analyze ALND patients only, except for sentinel lymph node dissection (SLND) patients. If ALND was performed at a similar rate in the control group, the postoperative lymphedema is expected to have a higher prevalence, which would have helped to support the usefulness of ILR. Second, the retrospective design of our study could include bias, because it did not use a perfectly matched cohort. In this type of study analyzing a risk factor, the matched case-control design will make it more reliable. Last, since the diagnosis of lymphedema was based on the circumference measure and clinical judgement, we have not solved the issue of transient or subclinical lymphedema. Our surveillance method with limb circumference measurement may include the bias in intrarater and interrater reliability. If the objective evaluation tools such as perometry and definite criteria of postoperative lymphedema are established, it is expected that more reliable results will be derived.
Conclusions
Prevention is the most important treatment for a refractory disease such as lymphedema. Our study suggested that ILR may be a promising surgical treatment to prevent postoperative lymphedema with borderline results. We believe that this procedure may serve as an effective option for prevention, especially in high-risk patients who are planning to undergo ALND and radiotherapy. There is a need for larger studies with longer follow-up to confirm the findings obtained in our study.
Acknowledgments
Funding: This research was supported by the Korea University (grant number K2225591) and the National Research Foundation of Korea (NRF) founded by the Ministry of Education (grant number R2113691).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-554/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-554/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-554/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Korea University Anam Hospital (protocol number 2021AN0524). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
1, all statistical analyses were performed in consultation with an independent medical statistician.
References
- Chung JH, Hwang YJ, Park SH, et al. Preliminary outcomes of combined surgical approach for lower extremity lymphedema: supraclavicular lymph node transfer and lymphaticovenular anastomosis. J Plast Surg Hand Surg 2022;56:261-9. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol 2009;16:703-8. [Crossref] [PubMed]
- Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol 2011;18:2500-5. [Crossref] [PubMed]
- Feldman S, Bansil H, Ascherman J, et al. Single Institution Experience with Lymphatic Microsurgical Preventive Healing Approach (LYMPHA) for the Primary Prevention of Lymphedema. Ann Surg Oncol 2015;22:3296-301. [Crossref] [PubMed]
- Chang DW, Dayan J, Greene AK, et al. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plast Reconstr Surg 2021;147:975-93. [Crossref] [PubMed]
- Cakmakoglu C, Shah H, Valente S, et al. Immediate Lymphatic Reconstruction After Axillary Lymphadenectomy Makes a Difference: Bioimpendence Spectroscopy and 2-Year Follow-up Analysis. Plast Reconstr Surg Glob Open 2020;8:73.
- Cook JA, Sasor SE, Loewenstein SN, et al. Immediate Lymphatic Reconstruction after Axillary Lymphadenectomy: A Single-Institution Early Experience. Ann Surg Oncol 2021;28:1381-7. [Crossref] [PubMed]
- Weinstein B, Le NK, Robertson E, et al. Reverse Lymphatic Mapping and Immediate Microsurgical Lymphatic Reconstruction Reduces Early Risk of Breast Cancer-Related Lymphedema. Plast Reconstr Surg 2022;149:1061-9. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery 2014;34:421-4. [Crossref] [PubMed]
- Jørgensen MG, Toyserkani NM, Sørensen JA. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: a systematic review and meta-analysis. Microsurgery 2018;38:576-85. [Crossref] [PubMed]
- Depypere B, Vyncke T, Dhooghe N, et al. A Novel Technique for Preventive Lymphovenous Anastomosis: Anastomosing a Ligated Lymphatic Vessel. Plast Reconstr Surg Glob Open 2021;9:e3509. [Crossref] [PubMed]
- Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol 2007;14:1890-5. [Crossref] [PubMed]
- Pressman PI. Surgical treatment and lymphedema. Cancer 1998;83:2782-7. [Crossref] [PubMed]
- Sitzia J. Volume measurement in lymphoedema treatment: examination of formulae. Eur J Cancer Care (Engl) 1995;4:11-6. [Crossref] [PubMed]
- Johnson AR, Fleishman A, Granoff MD, et al. Evaluating the Impact of Immediate Lymphatic Reconstruction for the Surgical Prevention of Lymphedema. Plast Reconstr Surg 2021;147:373e-81e. [Crossref] [PubMed]
- Huang YY, Toh PY, Hunt C, et al. Breast cancer treatment-related arm lymphoedema and morbidity: A 6-year experience in an Australian tertiary breast centre. Asia Pac J Clin Oncol 2022;18:109-17. [Crossref] [PubMed]
- Gowda AU, Nie J, Mets E, et al. Risk Factors for Lymphedema After Breast Conservation Therapy and Oncoplastic Reduction. Ann Plast Surg 2021;87:248-52. [Crossref] [PubMed]
- Johnson AR, Kimball S, Epstein S, et al. Lymphedema Incidence After Axillary Lymph Node Dissection: Quantifying the Impact of Radiation and the Lymphatic Microsurgical Preventive Healing Approach. Ann Plast Surg 2019;82:S234-41. [Crossref] [PubMed]
- Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer 2018;25:309-14. [Crossref] [PubMed]
- Nguyen TT, Hoskin TL, Habermann EB, et al. Breast Cancer-Related Lymphedema Risk is Related to Multidisciplinary Treatment and Not Surgery Alone: Results from a Large Cohort Study. Ann Surg Oncol 2017;24:2972-80. [Crossref] [PubMed]
- Schwarz GS, Grobmyer SR, Djohan RS, et al. Axillary reverse mapping and lymphaticovenous bypass: Lymphedema prevention through enhanced lymphatic visualization and restoration of flow. J Surg Oncol 2019;120:160-7. [Crossref] [PubMed]
- Guo X, Jiao D, Zhu J, et al. The effectiveness of axillary reverse mapping in preventing breast cancer-related lymphedema: a meta-analysis based on randomized controlled trials. Gland Surg 2021;10:1447-59. [Crossref] [PubMed]
- Hahamoff M, Gupta N, Munoz D, et al. A Lymphedema Surveillance Program for Breast Cancer Patients Reveals the Promise of Surgical Prevention. J Surg Res 2019;244:604-11. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008;26:5213-9. [Crossref] [PubMed]
- Rockson SG. Precipitating factors in lymphedema: myths and realities. Cancer 1998;83:2814-6. [Crossref] [PubMed]
- Ribeiro Pereira ACP, Koifman RJ, Bergmann A. Incidence and risk factors of lymphedema after breast cancer treatment: 10 years of follow-up. Breast 2017;36:67-73. [Crossref] [PubMed]
- Che Bakri NA, Kwasnicki RM, Khan N, et al. Impact of Axillary Lymph Node Dissection and Sentinel Lymph Node Biopsy on Upper Limb Morbidity in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Ann Surg 2023;277:572-80. [Crossref] [PubMed]
- Chung JH, Kim DW. Invited Response on: Commentary on "Aesthetic Comparison of Abdominal Donor Site Scar Between Absorbable Dermal Staple and Subcutaneous Suture after Autologous Breast Reconstruction: A Prospective Randomized Controlled, Double-Blinded Study". Aesthetic Plast Surg 2021;45:2544-5. [Crossref] [PubMed]
- Hackshaw A, Kirkwood A. Interpreting and reporting clinical trials with results of borderline significance. BMJ 2011;343:d3340. [Crossref] [PubMed]
- Chen WF, Knackstedt R. Delayed Distally Based Prophylactic Lymphaticovenular Anastomosis: Improved Functionality, Feasibility, and Oncologic Safety? J Reconstr Microsurg 2020;36:e1-2. [Crossref] [PubMed]


