
Potential factors affecting success rate and long term outcome in single balloon enteroscopy-assisted therapeutic endoscopic retrograde cholangiopancreatography in patients with pancreaticojejunal anastomotic stenosis: a retrospective study
Highlight box
Key findings
• Thicker pancreatic duct stents and long stent indwelling time can reduce the recurrence odds of PJS.
What is known and what is new?
• ERP is a safe alternative to treat PJS, although anastomotic site identification successful rate is relatively low.
• Late ERP intervention, digestive tract reconstruction method, pancreaticojejunostomy method, pancreatic duct tube placement during PD, pancreatic duct dilation before PD, and postoperative pancreatic fistula are potential factors affecting success rate.
What is the implication, and what should change now?
• Early endoscopic intervention should be carried once PJS occurs.
Introduction
With the progress of surgical techniques and the improvement in comprehensive treatment, the safety of pancreaticoduodenectomy (PD) and treatment effectiveness of malignant tumours have been significantly improved. Many patients have achieved long-term postoperative survival (1,2). Late complications after PD are common, and include bile duct stones, choledochojejunal anastomotic stenosis (CJS), pancreaticojejunal anastomotic stenosis (PJS), and recurrent pancreatitis (3), which seriously affect the quality of life of patients. Among these complications, PJS and chronic pancreatitis are difficult to treat, although relatively rare (4,5). Operative revision of the PJ is one of the main intervention to remedy the symptomatic and physiologic complications of this anastomotic stricture. Recently endoscopic treatments such as endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasonography-guided pancreatic drainage (EUS-PD) has been increasingly used as it has the advantages of being minimally invasive and repeatable, and has been shown to be superior to traditional surgical operations (6-8). However, effective treatment methods and standardized treatment strategies are still lacking. We conducted a retrospective study to analyse patients with PJS who were treated by therapeutic ERCP using single-balloon-assisted enteroscopy (BAE) at our centre over the past 5 years. We analysed and summarized the clinical data, diagnostic and treatment processes, and follow-up conditions with the goal of providing insights for the optimal diagnosis and treatment of such patients in the future. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-692/rc).
Methods
Patient selection
We retrospectively analyzed the clinical data and conducted follow-up of patients with PJS who underwent BAE-ERCP from March 2016 to March 2021 at Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and their following characteristics were evaluated: Gender, age, reason for surgery, operation course, perioperative time recovery information, PJS occurrence time and symptoms. Indications for BAE-ERCP included the following: (I) abdominal pain, emaciation, and other clinical symptoms consistent with chronic pancreatitis; (II) CT or MRCP indicating dilation of the pancreatic duct, with or without pancreatic duct stones; and (III) elevation in amylase levels three times higher than normal. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2022-044). Individual consent for this retrospective analysis was waived.
Enteroscopy and therapeutic equipment
We used SIF-260 single balloon enteroscope with a working length of 200 cm, a outer diameter of 9.2 mm and a 2.8 mm biopsy channel (Olympus Medical Systems, Tokyo, Japan). The sphincterotome or catheter with 320 cm length and a 600 cm guidewire (COOK, United States) were used for cannulation. Balloon dilator and 5 or 7 Fr pancreatic stent (Cook, United States) were also employed.
ERCP procedure
For the ERCP procedure, the anaesthesiology, digestive endoscopy, and surgery departments worked cooperatively to enhance the diagnostic and treatment success. The patient was placed in the supine position, and a ventilator was used to assist endotracheal intubation anaesthesia. SBE was performed by an endoscopist and a nurse who worked together to perform the operation. A CO2 supply was used.
First, the enteroscope was positioned near the gastrointestinal anastomotic site. During intubation, the endoscopist frequently checked under X-ray to move the scope towards the upper right abdomen and to ensure it was placed in the afferent loop. After the scope was positioned under the lower margin of the liver, the pancreaticojejunal anastomotic site was located, and cannulation was performed. For patients whose site was difficult to identify, the scope was carefully and slowly withdrawn after reaching the end of enteric cavity then repositioned. In the process of searching, the orientation and position of the endoscope tip was monitored by X-ray. Since the surface projection of the pancreatic duct opening is often located at the right side of the spine and the pancreatic duct is axially perpendicular to the spine, the search area could be narrowed to the loop where the scope direction and location were both concordant. For patients with many intestinal wall folds, a sphincterotome or catheter tip was used to gently lift the folds. After successful cannulation of the pancreatic duct, pancreatography was conducted, then, anastomotic dilation, pancreatic duct stone removal, or pancreatic duct stent placement was performed according to the diameter of the pancreatic duct and severity of PJS (Figure 1).
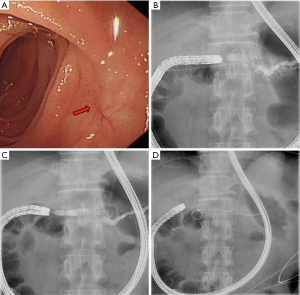
Outcome definitions and follow-up
Enteroscopy was considered successful when access to the pancreatic-enteric anastomotic site was achieved. Diagnostic success was defined as the acquisition of a pancreatogram, while treatment success was defined as completion of the intended intervention procedure. ERCP-related adverse events were categorized using ERCP consensus guidelines (9). Recurrence of pancreatic duct stenosis was defined as abdominal pain, pancreatic duct dilation, or the repeat elevation of amylase levels (10). All patients were followed up every 3 months to determine whether further ERCP was needed, and the end point of the follow-up was April 10, 2021, or the date of death.
Statistical analysis
Analyses were performed using SPSS version 23.0. The results are expressed as the median and interquartile range (IQR). Continuous variables were compared using Student’s t-test, and non-continuous variables were compared using Fisher’s exact test. A P value <0.05 was considered statistically significant.
Results
Patient demographics and clinical characteristics
A total of 16 patients underwent BAE-ERCP, comprising seven men and nine women, and their average age was 51 (range, 18–70) years. The detailed characteristics of patients are shown in Table 1. Surgical procedures included PD with Whipple reconstruction (n=11), PD with Child reconstruction (n=4), and pylorus-preserving pancreaticoduodenectomy (PpPD) with Whipple reconstruction (n=1). All patients had varying degrees of abdominal pain and weight loss, and abdominal CT showed abnormal manifestations, such as pancreatic duct thickening, peripancreatic exudation, or pancreatic duct stones.
Table 1
| Patients | N=16 |
|---|---|
| Sex (male/female) | 7/9 |
| Age (years), median [range] | 51 [18–70] |
| Primary disease | |
| Bile duct cancer | 1 |
| Pancreatic cancer | 3 |
| Ampullary cancer | 2 |
| Cystadenoma of the pancreas | 2 |
| SPNs of the pancreas | 2 |
| Duodenal malignant tumour | 3 |
| IPMN | 3 |
| Reconstruction methods | |
| PD with Whipple reconstruction | 11 |
| PD with Child reconstruction | 4 |
| PpPD with Whipple reconstruction | 1 |
| Symptom | |
| Abdominal pain | 16 |
| Fever | 2 |
| Diarrhoea | 4 |
| Weight loss | 16 |
| Diabetes | 3 |
| Imaging findings | |
| PD dilation | 9 |
| PD stone | 2 |
| Peripancreatic exudation | 6 |
SPNs, solid pseudopapillary neoplasms; IPMN, intraductal papillary mucinous neoplasm; PD, pancreaticoduodenectomy; PpPD, pylorus-preserving pancreaticoduodenectomy.
Treatment details and ERCP success rate
The treatment success rate and complication rate are shown in Table 2. Of all 16 patients, successful enteroscopic access to the intestinal loop where the pancreaticojejunal anastomotic site was located was achieved in 14 patients, with a scope intubation success rate of 87.5%, while two cases failed due to bending of the afferent loop. When the enteroscope was successfully placed, the pancreaticojejunal anastomotic sites of seven cases could be successfully identified, with a success rate of 50%. In one case, the initial ERCP failed to identify the anastomotic site, but EUS-PD + ERCP was successfully performed (Figure 2). Therefore, eight patients were successfully treated, and the overall success rate of the treatment was 50%. Among these eight patients, one underwent catheter dilation, seven underwent catheter or balloon dilation and then pancreatic duct stent placement (ERPD), and six patients received multiple ERCP treatments. The main goals of subsequent treatment included the assessment of anastomotic site dilation effectiveness or the replacement or removal of stents. All subsequent ERCP procedures were successful, and no patient experienced severe pancreatitis, gastrointestinal perforation, gastrointestinal bleeding, or other serious complications.
Table 2
| Outcome | Number (%) |
|---|---|
| Success rate (N=16) | |
| Enteroscopy success rate | 14 (87.5) |
| Diagnostic success rate | 8 (50.0) |
| Treatment success rate | 8 (50.0) |
| Number of ERCP procedures (N=8) | |
| Once | 2 (25.0) |
| Twice | 5 (62.5) |
| Three times | 1 (12.5) |
| Characteristics of the anastomotic site (N=8) | |
| Pinhole-like | 3 (37.5) |
| Split-like | 3 (37.5) |
| Membranous stenosis | 2 (25.0) |
| Cannulation (N=8) | |
| A combination of EUS-PD | 1 (12.5) |
| Enteroscope | 7 (87.5) |
| Intervention (N=8) | |
| Pre-cutting of anastomotic site | 1 (12.5) |
| Dilation of anastomotic site | 8 (100.0) |
| Dilating catheter | 6 (75.0) |
| Cylindrical balloon | 2 (25.0) |
| Extraction of PD stone | 1 (12.5) |
| Stent placement | 7 (87.5) |
| The initial placement of 5 Fr | 4 (50.0) |
| The initial placement of 7 Fr | 3 (37.5) |
| Replace 5 Fr with 7 Fr | 3 (37.5) |
| Stent removal | 3 (37.5) |
| Complications after ERCP | |
| Pancreatitis | 0 |
| Gastrointestinal perforation | 0 |
| Gastrointestinal bleeding | 0 |
ERCP, endoscopic retrograde cholangiopancreatography; EUS-PD, endoscopic ultrasonography-guided pancreatic drainage.
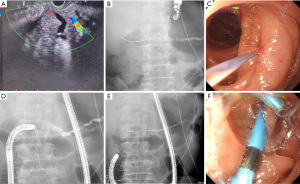
Analysis of risk factors for the failure of pancreaticojejunal anastomotic site identification
Of the 14 patients in whom enteroscopic entry was successful, seven achieved successful anastomotic site identification and there were seven cases of failure. The interval between the first occurrence of abdominal pain after PD operation in the successful group was significantly longer than in the failed group, while the time from symptom onset to the first ERCP intervention was shorter than in the failed group, with statistically significant differences (Table 3). The interval between PD surgery and the first ERCP intervention was not significantly different between the two groups. Univariate analyses were performed to evaluate the factors associated with anastomotic site identification failure, and the results showed this was related to the digestive tract reconstruction method, pancreaticojejunostomy method, pancreatic duct tube placement during PD, pancreatic duct dilation before PD, and postoperative pancreatic fistula (Table 4).
Table 3
| Factors | Successful anastomotic site identification | Failed anastomotic site identification | P |
|---|---|---|---|
| Time from the operation to the first abdominal pain event (months) | 44.71 | 19.86 | 0.044 |
| Time from the operation to the first ERCP intervention (months) | 53.14 | 42.29 | 0.372 |
| Time from symptom onset to the ERCP intervention (months) | 8.43 | 22.43 | 0.021 |
ERCP, endoscopic retrograde cholangiopancreatography.
Table 4
| Factors | Category | Successful anastomotic site identification | Failed anastomotic site identification | P |
|---|---|---|---|---|
| Age | <60 years | 3 | 3 | 1.000 |
| ≥60 years | 4 | 4 | ||
| Sex | Male | 4 | 3 | 0.606 |
| Female | 3 | 4 | ||
| Primary disease (benign or malignant) | Benign | 4 | 4 | 1.000 |
| Malignant | 3 | 3 | ||
| Digestive tract reconstruction method | Whipple | 7 | 3 | 0.001 |
| Child | 0 | 4 | ||
| Method of pancreaticojejunostomy | Invagination | 4 | 0 | 0.001 |
| Duct to mucosa | 3 | 7 | ||
| Pancreatic duct dilation before PD | Yes | 6 | 2 | 0.037 |
| No | 1 | 5 | ||
| Pancreatic duct tube placement during PD | Yes | 6 | 2 | 0.037 |
| No | 1 | 5 | ||
| Postoperative pancreatic fistula | Yes | 1 | 6 | 0.010 |
| No | 6 | 1 | ||
| Pancreatic duct dilation before ERCP | Yes | 3 | 3 | 1.000 |
| No | 4 | 4 | ||
| Pancreatic duct stones | Yes | 1 | 2 | 0.530 |
| No | 6 | 5 | ||
| AMY increases before ERCP | Yes | 5 | 4 | 0.591 |
| No | 2 | 3 |
PD, pancreaticoduodenectomy; ERCP, endoscopic retrograde cholangiopancreatography; AMY, amylase.
Subsequent treatment and follow-up
The median follow-up time of the eight patients in whom ERCP treatment was successful was 77.2 months (IQR, 6.8–187.7 months), and the mean indwelling time of the stent in seven of these patients was 62.3 months (IQR, 6.8–153.7 months). The follow-up results are shown in Table 5. The BMI of all patients who received successful clinical treatment increased significantly in the years after ERP. Patients who experienced treatment failure or recurrence of PJS all suffered different degrees of weight loss, while the BMI in one patient was found to have increased by 1.12 within 16 months after the initial successful treatment. However, after recurrence, his BMI decreased by 1.46 within 3 months. The detailed treatment process of all patients is shown in Table 6. Among the two patients who experienced recurrence of pancreatitis, the first only underwent dilation of the pancreaticojejunal anastomotic site without stent implantation during the ERP process. However, the pancreatitis recurred 2 months later, and the patient then chose surgical treatment. The second patient underwent anastomotic dilation and 5-Fr pancreatic duct stent implantation for the first ERP, and his pancreatitis symptoms were initially relieved. However, 16 months later, he requested stent removal and refused to replace it with a 7-Fr stent, and pancreatitis again recurred one month later. Remedial ERP to insert another stent after another 2 months was unsuccessful due to failure of anastomotic site identification, and he finally chose surgery but died of postoperative bleeding. Among the remaining six patients who did not experience recurrence, two underwent ERP with implantation of 7-Fr stents, which have been retained to date; two patients underwent another ERP procedure to replace the 5-Fr stent with a 7-Fr stent after more than one year, which have been retained to date; and two patients underwent 7-Fr stent implantation after more than one year, and no recurrence occurred after stent removal.
Table 5
| Characteristic | Value |
|---|---|
| Median follow-up time (n=8, months) | 77.2 (6.8–187.7) |
| Stent indwelling time (n=7, months) | 62.3 (6.8–153.7) |
| Stent displacement (yes/no) | 0/7 |
| Stent congestion (yes/no) | 0/7 |
| Stent removal (yes/no) | 3/4 |
| Relapse (yes/no) | 2/6 |
| Recurrence time after last ERCP (n=2, months) | 1.1 |
| Treatment for recurrent patients (surgery/ERCP/conservative) | 2/0/0 |
| Treatment results (success/failure) | 0/2 |
| BMI variation | |
| Non-recurrence group (n=6) | 2.46 |
| Recurrence group (n=2) | −1.09 |
| ERCP treatment failure group (n=8) | −2.12 |
ERCP, endoscopic retrograde cholangiopancreatography; BMI, body mass index.
Table 6
| Patient No. | Number of ERCP treatments | Duration and course of ERCP treatment | Follow-up time (month) | Relapse or not | Time of recurrence after the last ERCP (month) | Stent indwelling time (month) | BMI change |
|---|---|---|---|---|---|---|---|
| A | 1 | 2018.1; 5-Fr dilation catheter tip dilated anastomotic site | 39.0 | Yes | 1.0 | 0.0 | −1.81 |
| B | 2 | 2016.8; 5-Fr stent was inserted after 5-Fr catheter dilation | 20.2 | Yes | 1.2 | 15.7 (5 Fr) | −0.34 |
| 2017.11; removal of pancreatic duct stent | |||||||
| C | 2 | 2019.6; 5-Fr catheter dilation, followed by placement of a 7-Fr stent | 21.8 | No | – | 13.3 (7 Fr) | +3.9 |
| 2020.7; removal of the pancreatic duct stent | |||||||
| D | 2 | 2019.11; 4 mm cylindrical balloon dilated anastomotic site + 5-Fr stent placement | 17.7 | No | – | 15 (5 Fr) + 2.7 (7 Fr) | +0.32 |
| 2021.2; 4 mm cylindrical balloon dilated anastomotic site + placement of 7-Fr stent | |||||||
| E | 2 | 2020.12; anastomotic site could not be identified | 2.7 | No | – | 2.7 (7 Fr) | 0.00 |
| 2021.1; cannulation was successful with a combination of enteroscopy and EUS-PD; the anastomotic was dilated with a 7-Fr dilation catheter, and a 7-Fr stent was inserted, which has been retained to date | |||||||
| F | 1 | 2016.3; a 7-Fr stent was inserted after dilation, which has been retained to date | 61.5 | No | – | 61.5 (7 Fr) | +6.76 |
| G | 3 | 2015.2; the anastomotic site was dilated with a 5-Fr catheter, and 5-Fr stent was inserted | 75.1 | No | 29.8 (5 Fr) + 25.5 (7 Fr) | +2.00 | |
| 2017.8; the anastomotic site was dilated with a 7-Fr catheter, and a 7-Fr stent was inserted | – | ||||||
| 2019.8; removal of the pancreatic duct stent | |||||||
| H | 2 | 2020.7; 6 mm cylindrical balloon dilated anastomotic site + partial stone extraction + 5-Fr stent placement | 9.0 | No | – | 6 (5 Fr) + 3 (7 Fr) | +1.83 |
| 2021.1; 6 mm cylindrical balloon dilation anastomotic site + removal of all stones + placement of 7-Fr stent, which has been retained to date |
ERCP, endoscopic retrograde cholangiopancreatography; BMI, body mass index.
Discussion
According to research, the success rate of endoscopic treatment for bile duct-related disease after PD is relatively high, at approximately 50–94% (11-14). However, the success rate of endoscopic treatment for pancreatic diseases is only 8–38% (15-17), because the pancreaticojejunal anastomotic site is difficult to identify and pancreatic duct cannulation is challenging. Repeatability is one of the advantages making endoscopic treatment better than traditional surgery, and in this study, the success rate of BAE-ERCP treatment was 50%, which was superior to the results reported in relevant articles (18,19), with six patients undergoing successful treatment multiple times. The first ERCP intervention was beneficial for confirming the digestive tract structure and for dilating the pancreaticojejunal anastomotic site, which made subsequent ERCP procedures easier to perform. No severe complications occurred in our study, including gastrointestinal perforation, acute major pancreatitis, or gastrointestinal bleeding. Two patients elected to undergo surgery after ERCP failure or the reoccurrence of pancreatitis, and one suffered from intra-abdominal haemorrhage after surgery, which resulted in his death. While one article reported the overall morbidity rate after PJ revision was 26% (20), in contrast, ERCP has the advantages of minimal invasiveness and high safety. As an unsuccessful endoscopic attempt will not cause trauma or affect the implementation of other treatments, it can be used as the first choice for centres with extensive endoscopic experience and a full set of equipment.
Pancreaticojejunal anastomotic site identification is one of the biggest challenges of this procedure, and has been categorized into three types: pinhole-like, split-like, and membranous stenosis (6). Among these, the pinhole and split-like openings are easier to identify, while membranous stenosis openings are often accompanied by atresia of the anastomotic site (Figure 3). When the anastomotic site cannot be identified under the enteroscope, indirect signs should be considered instead, such as changes in intestinal mucosal continuity or the formation of scar tissue. In some cases, the physician might need to observe the intestinal wall for a long duration to capture the moment when a small amount of secreted pancreatic fluid flows. Cannulation of several suspicious depressions can then be attempted after further narrowing the area of the pancreatic duct under enteroscopic visualization. When cannulating, the sphincterotome or a catheter can be used to access the possible opening position of the pancreatic duct, and the assistant can use the tip of the guidewire to gently tap the depression. If the tip can be pushed deep, the assistant can continue to penetrate the guidewire under X-ray, and penetration to a depth of approximately 5 cm outside the contour of the intestinal cavity without resistance usually indicates it has successfully entered the pancreatic duct. At this time, the physician can insert the sphincterotome or catheter into the opening and inject contrast agent to determine whether the pancreatic duct is developed. During the cannulation attempt, forcing the sphincterotome or the tip of the catheter against the mucosa should be avoided, and the assistant must also avoid rough penetration with the guidewire. These steps can easily cause oedema or bleeding of the intestinal mucosa so that the pancreaticojejunal anastomotic site, which is originally difficult to identify, disappears into the swollen mucosa, leading to the subsequent failure of enteroscopic treatment. Contrast agent should also not be injected in these circumstances. For anastomotic sites that cannot be identified by the methods above, methylene blue could be used to dye the intestinal wall, or patients can be administered a pancreatic secretion accelerator before surgery to improve the success rate of identification (21). In addition, EUS-PD is a common alternative, with a reported success rate of 50–100% (22-24). In this study, the anastomotic site could not be identified by enteroscopy in one patient, but pancreatic duct stent implementation was performed successfully by EUS-PD, and satisfactory treatment results were achieved. However, the success rate of this technically difficult method is closely related to the dilation diameter of the pancreatic duct, and a complication rate of 5–35% has been reported (25,26), including serious complications such as gastrointestinal perforation and abdominal bleeding. Due to the limited number of cases reported in the relevant literature, the effectiveness and safety of this method require further study.
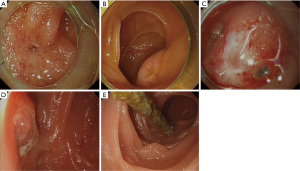
In this study, we analysed the factors associated with anastomotic site identification failure in 14 patients whose pancreatic intestinal anastomotic site area could be successfully reached by endoscopy. We found for the first time that the failure of anastomotic site identification was related to the method of gastrointestinal reconstruction, the appearance of the anastomotic site, the placement of a pancreatic duct support tube during PD, the diameter of the pancreatic duct before PD, the occurrence of pancreatic fistula after PD, and the occurrence time and first endoscopic intervention time of postoperative pancreatitis. According to digestive tract reconstruction methods, the anastomotic site is more difficult to identify during the Child procedure than during the Whipple procedure. This is because for Whipple cases, the anastomotic site is close to the end of the afferent loop, so when the enteroscope enters, the axial direction of the pancreatic duct is parallel to the endoscope, and the opening of the duct is in the middle of view. In Child cases, it is difficult for the operator to estimate the position of the intestinal segment where the anastomotic site should be, and the axial direction of the pancreatic duct is perpendicular to the endoscope, which makes the site at the edge of view and difficult to find. In this study, the anastomotic site could not be identified under endoscopy in all four patients who underwent the Child reconstruction method. The diameter of the pancreatic duct and pancreaticojejunostomy method during PD are also risk factors affecting the success rate of endoscopic treatment. Thin pancreatic ducts and pancreaticojejunal mucosa-to-mucosa anastomosis during PD make reconstruction difficult and increase postoperative pancreatic fistula rates. Once pancreatic fistula occurs after PD, local inflammatory stimulation is induced, which often leads to pancreaticojejunostomy stricture, or the anastomotic site becomes covered by mucosa. If the pancreatic duct support tubes are not placed during PD, the possibility of pancreatic fistula will increase, or early anastomotic site collapse will occur. In this study, we also found that the time to the first pancreatitis event after PD and the ERCP intervention time were significantly different. The early occurrence of pancreatitis is often related to defective anastomosis or poor healing of the anastomotic site, and the lesion becomes covered by inflammatory hypertrophic scar tissue, complicating subsequent ERCP treatment. On the other hand, with the passage of time after the first pancreatitis event, the more severe the pancreaticointestinal anastomotic site stenosis level could be when subsequent ERCP intervention is conducted. When needle-like stenosis or complete atresia is observed, patients lose the opportunity for successful enteroscopic treatment, and the risk of stenosis recurrence increases. Therefore, we suggest patients with postoperative pancreatitis, especially those with symptoms at early time points after surgery, should be treated with ERCP as early as possible to maximize the clinical benefit of enteroscopic treatment.
In this study, we revealed for the first time that a change in BMI is closely related to the efficacy of ERCP and the recurrence of pancreatitis. We hope that with the inclusion of more cases in later phases, a scoring system can be established to evaluate the effectiveness of ERP treatment and to predict whether pancreatitis relapse will occur. Based on patient follow-up, we summarized several preliminary findings and hope to motivate further research to determine optimal ERCP treatment strategies: (I) patients who underwent only pancreaticojejunal anastomotic site dilatation without stent implantation relapsed early after surgery. (II) The need for pancreaticojejunal anastomotic site dilatation and catheter or balloon dilatation are not directly related to the recurrence of pancreatitis, which is also consistent with other research results (27). (III) No patient experienced pancreatitis recurrence during stent indwelling. Similar studies have shown that stent detachment after ERP is the only risk factor for the recurrence of pancreatitis (7,28). (IV) One patient with anastomotic site dilatation and a 5-Fr pancreatic duct stent placed for one-year experienced recurrent pancreatitis in a short time after stent removal, suggesting the expansion effect of a 5-Fr stent on the anastomotic site may be insufficient. (V) Cases with a 7-Fr stent placed for more than one year did not experience pancreatitis recurrence after stent removal. According to the above findings, we believe the effective expansion of the anastomotic site with stents is the key to ensuring treatment efficacy (Figure 4). Therefore, we suggest that during ERCP treatment, thicker pancreatic duct stents should be placed, and if only 5-Fr stents can be placed during ERP for the first time due to severe pancreatic duct stenosis, 7-Fr stents should replace them in subsequent endoscopic treatment. In this study, no stent occlusion was observed. In some studies stent indwelling time longer than six months may result in stent obstruction and pancreatitis (29,30). However, in PJS cases, stent obstruction rate is relatively low even the patients experienced a long-term or permanent stent placement (8). And based on this, we do not recommend pancreatic duct stents be removed. For patients who have a strong desire for stent removal, it is recommended the 7-Fr stent be retained for at least one year before removal. When removing the stents, we recommend careful visualization of the appearance of the anastomotic site or to fill it with a small-diameter dilation balloon to observe whether there is a narrow ring at the site. If anastomotic site stenosis still exists, stent reinsertion is recommended, which should be retained for at least another year.
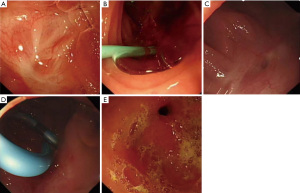
This study has several limitations. First, this was a single-centre retrospective study, and second, the small number of cases meant some statistical analyses were difficult to conduct. In addition, the follow-up time of some patients was relatively short, and finally, there was no control group.
Conclusions
This study preliminarily verified the safety and effectiveness of enteroscopy ERCP treatment for PJS after PD, proposed operative techniques, and identified risk factors for pancreaticojejunal anastomotic site identification failure for the first time. Our results show ERCP intervention should be carried out early if chronic pancreatitis caused by PJS occurs, and BMI is an important index to be monitored during the follow-up of such patients. The use of thicker pancreatic duct stents over a long period of time to reduce the recurrence odds of anastomotic stenosis is recommended.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (Grant/Award Number: 82272691).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-692/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-692/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-692/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2022-044). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolfgang CL, Pawlik TM. Pancreaticoduodenectomy: time to change our approach? Lancet Oncol 2013;14:573-5. [Crossref] [PubMed]
- Nassour I, Choti MA. Pancreatic Operations. JAMA 2016;316:1932. [Crossref] [PubMed]
- Regimbeau JM, Rebibo L, Dokmak S, et al. The short- and long-term outcomes of pancreaticoduodenectomy for cancer in Child A patients are acceptable: a patient-control study from the Surgical French Association report for pancreatic surgery. J Surg Oncol 2015;111:776-83. [Crossref] [PubMed]
- Zarzavadjian Le Bian A, Cesaretti M, Tabchouri N, et al. Late Pancreatic Anastomosis Stricture Following Pancreaticoduodenectomy: a Systematic Review. J Gastrointest Surg 2018;22:2021-8. [Crossref] [PubMed]
- Demirjian AN, Kent TS, Callery MP, et al. The inconsistent nature of symptomatic pancreatico-jejunostomy anastomotic strictures. HPB (Oxford) 2010;12:482-7. [Crossref] [PubMed]
- Kida A, Shirota Y, Houdo Y, et al. Endoscopic characteristics and usefulness of endoscopic dilatation of anastomotic stricture following pancreaticojejunostomy: case series and a review of the literature. Therap Adv Gastroenterol 2016;9:913-9. [Crossref] [PubMed]
- Kogure H, Sato T, Nakai Y, et al. Endoscopic management of pancreatic diseases in patients with surgically altered anatomy: clinical outcomes of combination of double-balloon endoscopy- and endoscopic ultrasound-guided interventions. Dig Endosc 2021;33:441-50. [Crossref] [PubMed]
- Kida A, Shirota Y, Kawane T, et al. Long-term outcomes after endoscopic retrograde pancreatic drainage for symptomatic pancreaticojejunal anastomotic stenosis. Sci Rep 2021;11:4489. [Crossref] [PubMed]
- Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383-93. [Crossref] [PubMed]
- Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446-54. [Crossref] [PubMed]
- Mönkemüller K, Fry LC, Bellutti M, et al. ERCP with the double balloon enteroscope in patients with Roux-en-Y anastomosis. Surg Endosc 2009;23:1961-7. [Crossref] [PubMed]
- Itoi T, Ishii K, Sofuni A, et al. Single-balloon enteroscopy-assisted ERCP in patients with Billroth II gastrectomy or Roux-en-Y anastomosis (with video). Am J Gastroenterol 2010;105:93-9. [Crossref] [PubMed]
- Siddiqui AA, Chaaya A, Shelton C, et al. Utility of the short double-balloon enteroscope to perform pancreaticobiliary interventions in patients with surgically altered anatomy in a US multicenter study. Dig Dis Sci 2013;58:858-64. [Crossref] [PubMed]
- Katanuma A, Yane K, Osanai M, et al. Endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy using balloon-assisted enteroscope. Clin J Gastroenterol 2014;7:283-9. [Crossref] [PubMed]
- Menon KV, Sanaka M. Successful single-balloon enteroscopic dilation of late anastomotic pancreaticojejunostomy stricture following whipple procedure. Pancreas 2010;39:115-6. [Crossref] [PubMed]
- Kikuyama M, Itoi T, Ota Y, et al. Therapeutic endoscopy for stenotic pancreatodigestive tract anastomosis after pancreatoduodenectomy (with videos). Gastrointest Endosc 2011;73:376-82. [Crossref] [PubMed]
- Park JH, Ye BD, Byeon JS, et al. Approaching pancreatic duct through pancreaticojejunostomy site with double ballon enteroscope in patients with Roux-en-Y anatomy. Hepatogastroenterology 2013;60:1753-8.
- Shimatani M, Matsushita M, Takaoka M, et al. Effective "short" double-balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy 2009;41:849-54. [Crossref] [PubMed]
- Farrell J, Carr-Locke D, Garrido T, et al. Endoscopic retrograde cholangiopancreatography after pancreaticoduodenectomy for benign and malignant disease: indications and technical outcomes. Endoscopy 2006;38:1246-9. [Crossref] [PubMed]
- Cioffi JL, McDuffie LA, Roch AM, et al. Pancreaticojejunostomy Stricture After Pancreatoduodenectomy: Outcomes After Operative Revision. J Gastrointest Surg 2016;20:293-9. [Crossref] [PubMed]
- Kin T, Takahashi K, Katanuma A. Successful balloon enteroscope-guided pancreatic ductal stenting of stricture at pancreaticojejunal anastomosis using chromoendoscopy with indigo carmine. Dig Endosc 2020;32:e1-2. [Crossref] [PubMed]
- Widmer J, Sharaiha RZ, Kahaleh M. Endoscopic ultrasonography-guided drainage of the pancreatic duct. Gastrointest Endosc Clin N Am 2013;23:847-61. [Crossref] [PubMed]
- Ergun M, Aouattah T, Gillain C, et al. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy 2011;43:518-25. [Crossref] [PubMed]
- Hodo Y, Shirota Y, Suda T, et al. Successful EUS-guided retrograde pancreatic duct stent placement for refractory pancreaticojejunostomy stricture after pancreaticoduodenectomy with a forward-viewing echoendoscope. VideoGIE 2018;3:196-8. [Crossref] [PubMed]
- Moreels TG. Endoscopic retrograde cholangiopancreatography in patients with altered anatomy: How to deal with the challenges? World J Gastrointest Endosc 2014;6:345-51. [Crossref] [PubMed]
- Takikawa T, Kanno A, Masamune A, et al. Pancreatic duct drainage using EUS-guided rendezvous technique for stenotic pancreaticojejunostomy. World J Gastroenterol 2013;19:5182-6. [Crossref] [PubMed]
- Sano I, Katanuma A, Kuwatani M, et al. Long-term outcomes after therapeutic endoscopic retrograde cholangiopancreatography using balloon-assisted enteroscopy for anastomotic stenosis of choledochojejunostomy/pancreaticojejunostomy. J Gastroenterol Hepatol 2019;34:612-9. [Crossref] [PubMed]
- Matsubayashi H, Kishida Y, Shinjo K, et al. Endoscopic ultrasound-guided retrograde pancreatic stent placement for the treatment of stenotic jejunopancreatic anastomosis after a Whipple procedure. Endoscopy 2013;45 Suppl 2 UCTN:E435-6.
- Morgan DE, Smith JK, Hawkins K, et al. Endoscopic stent therapy in advanced chronic pancreatitis: relationships between ductal changes, clinical response, and stent patency. Am J Gastroenterol 2003;98:821-6. [Crossref] [PubMed]
- Smits ME, Badiga SM, Rauws EA, et al. Long-term results of pancreatic stents in chronic pancreatitis. Gastrointest Endosc 1995;42:461-7. [Crossref] [PubMed]
(English Language Editor: B. Draper)


