Desmoid tumor following abdominally-based free flap breast reconstruction
Introduction
Desmoid tumors are benign, locally-aggressive, fibrous tumors that arise from musculoaponeurotic structures. Desmoid tumors most commonly develop in the rectus abdominis muscle and/or fascia and surgical scars of the abdominal wall in young women (1). Intra-abdominal forms commonly develop within the small bowel mesentery (2,3). Genetic predispositions to desmoid tumors throughout the body including the breast have been described (4). Gardner’s syndrome is a hereditary condition associated with the development of colonic polyposis, osteomas, and desmoid tumors that should always be considered in these patients (5).
Desmoids of the breast and chest wall are very uncommon and are believed to arise from the pectoralis major muscle fascia or Cooper’s ligaments (2). They have been described following various breast operations, including excisional biopsies, lumpectomies, mastectomies, breast reductions, and augmentations (6-9). In the setting of breast implants, desmoid tumors have been hypothesized to arise from the fibrous capsule around the prosthesis (7). However, development of a desmoid tumor following autologous breast reconstruction has not been described. A patient who developed a desmoid tumor of the breast/chest wall following abdominally-based free flap breast reconstruction is presented in the following report.
Case presentation
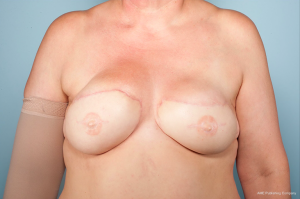
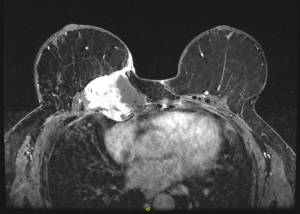
A 47-year-old female with a history of left breast carcinoma treated with breast conservation therapy developed right breast invasive ductal carcinoma and underwent bilateral mastectomies with immediate tissue expander reconstruction at an outside institution. She underwent radiation therapy and subsequently developed infections requiring expander removal. The patient presented to the Mayo Clinic, Rochester for autologous reconstruction. Delayed, bilateral muscle-sparing transverse rectus abdominis musculocutaneous (TRAM) flaps were performed. The immediate postoperative course was uneventful. At two year follow-up, physical examination revealed a firm, fixed mass at the superomedial aspect of her right breast (Figure 1). MRI revealed a 5.9 cm × 6.8 cm × 7.9 cm mass within the flap involving the pectoralis major muscle between the third and fourth anterior ribs with possible invasion of the parietal pleura (Figure 2). Core needle biopsy revealed desmoid fibromatosis.
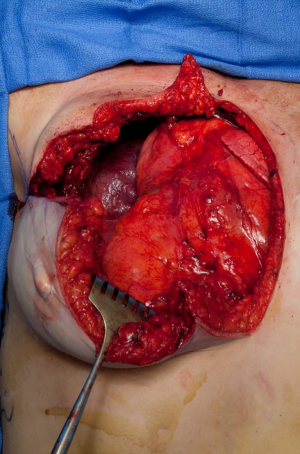
The patient underwent a wide local resection of the right anterior chest wall desmoid, including a portion of the flap, a majority of the sternum, bilateral internal mammary vessels including the microvascular anastomoses, and ipsilateral ribs 2–5 (Figure 3). Temporary chest wall closure was accomplished with polyglycolic acid mesh and re-inset of the remaining flap to the chest wall. The surgical margins were found to be negative for tumor. She underwent hyperbaric oxygen therapy post-operatively due to ischemia of the flap after resection of the vascular pedicle. One week post-resection, reconstruction was performed with a Gore-Tex patch. The entirety of the remaining free flap on the right was nonviable despite the hyperbaric oxygen and resected. A large soft tissue defect of greater than 20 cm in diameter was covered with a pedicled latissimus dorsi musculocutaneous flap.
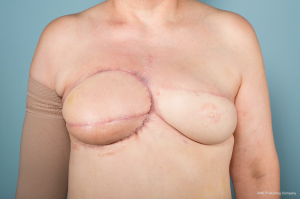
Approximately one year later, revision breast reconstruction began with a thoracoabdominal advancement flap, fat grafting, and revision of the latissimus dorsi flap. She underwent serial revision surgeries over the course of eleven months with a good final aesthetic outcome (Figure 4). She underwent close monitoring for tumor recurrence by chest CT scan every three months for the first year following resection and then at 6-month intervals with no recurrence. Genetic testing revealed a positive BRCA2 mutation. Family history and screening colonoscopies to evaluate for polyposis in association with Gardner’s syndrome were unremarkable.
Discussion
Desmoid tumors are very rare with a yearly incidence reported to be only 2 to 4 per million population (3). Histopathologic appearance is benign; however, they are locally aggressive with a high recurrence rate that requires early diagnosis and wide surgical resection (3). Desmoid tumors can occur in any anatomic location, but tumors of the breast are exceedingly rare. Breast desmoids usually present as a painless lump without discharge and are variable in size (10). There were several case reports published in the literature regarding desmoid tumor occurrence in the breast and chest wall (8,10-15). Desmoid tumors are more frequent in women than in men; hypotheses regarding possible etiology include associations with pregnancy and estrogen along with accidental or surgical trauma (15).
Some authors have suggested that silicone exposure in the setting of implant-based reconstruction could have been an inciting factor in the formation of such tumors (13). However, the development of a desmoid tumor may be associated with the surgical trauma of mastectomy prior to the placement of an implant rather than silicone exposure (13,15). Others have suggested that desmoid tumors may arise from the fibrous capsule that forms around the implant in patients who underwent breast augmentation (1,7). Jewett et al. showed histological continuity of the tumor with the fibrous capsule to support this hypothesis (7).
The inciting event leading to development of a desmoid tumor in the presented patient is difficult to ascertain. There are several possibilities to explain its etiology. The patient had a history of a mastectomy, infected expanders, and radiation which can each independently contribute to desmoid development. However, these factors were completed several years prior to the development of the desmoid. Considering the capsule theory, there is the possibility that a remnant of fibrous capsule from the expanders was left behind and was a nidus for tumor development. Surgical trauma of the abdominally-based free flap reconstruction may be the most likely explanation based on both the timing and anatomic location of tumor development. Considering the most common anatomic site for desmoid tumors is the abdominal wall, the most noteworthy possible source is the flap. There was no evidence of a mass within the abdominal wall on the CT angiogram obtained for preoperative microsurgical planning. Two radiologists independently reviewed the imaging and interpreted a potential desmoid epicenter superficial to the native chest wall within the TRAM flap, indicating likely origin from the flap rather than the chest wall. Desmoid tumors are cytologically bland on immunohistologic staining; they appear and stain similarly regardless of intra- or extra-abdominal location, so origin cannot be determined by histology alone (16-18). Identification of the exact anatomic origin of this patient’s desmoid as originating from the flap or chest wall is uncertain due to the tumor’s large size and extent of local invasion. It is possible that surgical trauma to the rectus abdominis muscle and fascia incited the cellular pathway that led to desmoid tumor development within the free flap that then flourished with its new recipient blood supply. The desmoid tumor was in the vicinity of the flap pedicle; therefore another possible explanation is that the additional surgical trauma of rib removal for anastomosis to the internal mammary vessels initiated development of the tumor from the chest wall. In either scenario, autologous reconstruction was believed to be the etiologic factor that led to the development of the desmoid tumor. Although this is the only case reported in the literature, plastic surgeons performing autologous breast reconstruction should be aware of this potential complication in patients with a genetic predisposition and/or increased risk of desmoid tumor.
Although this patient underwent numerous revision operations following tumor resection, a metachronous desmoid tumor did not develop in the latissimus dorsi muscle flap or its donor site. Similarly, a desmoid tumor did not develop in the reconstructed breasts following multiple sessions of fat grafting nor within the liposuction donor sites. This further suggests the possibility of abdominal wall etiology considering the predisposition for desmoid formation.
Conclusions
Desmoid tumors of the breast and chest wall are rare with several hypotheses regarding etiology. Although this is an unusual occurrence in the setting of abdominally-based free flap breast reconstruction, this case necessitates that desmoid tumor be included within the differential of a patient who presents with a breast mass following autologous reconstruction.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Schiller VL, Arndt RD, Brenner RJ. Aggressive fibromatosis of the chest associated with a silicone breast implant. Chest 1995;108:1466-8. [Crossref] [PubMed]
- Godwin Y, McCulloch TA, Sully L. Extra-abdominal desmoid tumour of the breast: review of the primary management and the implications for breast reconstruction. Br J Plast Surg 2001;54:268-71. [Crossref] [PubMed]
- Méndez-Fernández MA, Gard DA. The desmoid tumor: "benign" neoplasm, not a benign disease. Plast Reconstr Surg 1991;87:956-60. [PubMed]
- Zayid I, Dihmis C. Familial multicentric fibromatosis--desmoids. A report of three cases in a Jordanian family. Cancer 1969;24:786-95. [Crossref] [PubMed]
- Haggitt RC, Booth JL. Bilateral fibromatosis of the breast in Gardner's syndrome. Cancer 1970;25:161-6. [Crossref] [PubMed]
- Hammoudeh ZS, Darian VB. Desmoid tumor (fibromatosis) of the breast after augmentation with saline implants. Plast Reconstr Surg 2012;129:753e-4e. [Crossref] [PubMed]
- Jewett ST Jr, Mead JH. Extra-abdominal desmoid arising from a capsule around a silicone breast implant. Plast Reconstr Surg 1979;63:577-9. [Crossref] [PubMed]
- Mátrai Z, Tóth L, Gulyás G, et al. A desmoid tumor associated with a ruptured silicone breast implant. Plast Reconstr Surg 2011;127:1e-4e. [Crossref] [PubMed]
- Khanfir K, Guinebretiere JM, Vanel D, et al. Unusual problems in breast cancer and a rare lung cancer case. Case 2. Aggressive fibromatosis of the chest wall arising near a breast prosthesis. J Clin Oncol 2003;21:2216-8. [Crossref] [PubMed]
- Brown CS, Jeffrey B, Korentager R, et al. Desmoid tumors of the bilateral breasts in a patient without Gardner syndrome: a case report and review of literature. Ann Plast Surg 2012;69:220-2. [Crossref] [PubMed]
- Chummun S, McLean NR, Abraham S, et al. Desmoid tumour of the breast. J Plast Reconstr Aesthet Surg 2010;63:339-45. [Crossref] [PubMed]
- Crestinu JM. Desmoid tumor of the breast. Plast Reconstr Surg 1995;95:421. [Crossref] [PubMed]
- Dale PS, Wardlaw JC, Wootton DG, et al. Desmoid tumor occurring after reconstruction mammaplasty for breast carcinoma. Ann Plast Surg 1995;35:515-8. [Crossref] [PubMed]
- Vandeweyer E, Deraemaecker R. Desmoid tumor of the breast after reconstruction with implant. Plast Reconstr Surg 2000;105:2627-8. [Crossref] [PubMed]
- Dashiell TG, Payne WS, Hepper NG, et al. Desmoid tumors of the chest wall. Chest 1978;74:157-62. [Crossref] [PubMed]
- Cates JM, Black J, Wolfe CC, et al. Morphologic and immunophenotypic analysis of desmoid-type fibromatosis after radiation therapy. Hum Pathol 2012;43:1418-24. [Crossref] [PubMed]
- Goldstein JA, Cates JM. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol 2015;22:260-6. [Crossref] [PubMed]
- Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol 2006;28:105-11. [Crossref] [PubMed]


