
Effect of the HER-2/CEP17 ratio in IHC 2+/FISH-amplified breast cancer on pathological complete response to neoadjuvant pertuzumab and trastuzumab treatment—a retrospective cohort study
Highlight box
Key findings
• Our study finds that a HER-2/CEP17 ratio ≥6.0 might be related to more achievement of pCR in the neoadjuvant anti-HER-2 dual-targeted therapies.
What is known and what is new?
• The results of our real-world study show that a HER2/CEP17 ratio ≥6.0 in patients with IHC 2+/FISH-amplified early breast cancer who received neoadjuvant dual targeting is associated with a significantly higher pCR rate.
• Our study adds to the evidence of HER2/CEP17 ratio as a predictor of response to neoadjuvant dual target therapy in patients with IHC 2+/FISH-amplified breast cancer.
What is the implication, and what should change now?
• Therefore, our findings have important implications for treatment and clinical management decisions in this type of breast cancer.
Introduction
Breast cancer is an aggressive malignant tumor, with a high mortality and morbidity rate worldwide. An increase in the expression of human epidermal growth factor receptor-2 (HER-2) is observed in 15–20% of breast cancer cases, and therefore serves as a clinical diagnostic marker for breast cancer (1,2). The clinical application of anti-HER-2 drugs, such as trastuzumab, has significantly improved the survival of patients with HER-2-positive breast cancer (3). However, despite this progress, the prognosis of HER-2-positive breast cancer remains poor. Recently, the introduction of HER-2 dual-targeted therapies, such as the combination of trastuzumab and pertuzumab, has significantly improved invasive disease-free survival (4). HER-2 has been considered an important clinical marker for assessing the efficacy of drugs for treating HER-2-positive breast cancer. However, the status of HER-2 in response to clinical treatment with neoadjuvant pertuzumab and trastuzumab is unclear.
Chemotherapy is widely used to treat locally advanced breast cancer in early-stage patients, as it significantly increases the pathological complete response (pCR), which is an independent parameter that indicates the complete disappearance of invasive cancer (5). Several studies have shown that chemotherapy combined with neoadjuvant HER-2 dual-targeted therapy has better pCR and survival rates than therapy with neoadjuvant trastuzumab alone (5-7). The KATHERINE study showed that in patients with residual tumors after chemotherapy for HER-2-amplified breast cancer, adjuvant t-DM1 enhancement significantly reduced the risk of recurrence and improved survival compared with trastuzumab monotherapy (8). Therefore, understanding the clinicopathological changes that lead to higher pCR rates in patients receiving neoadjuvant chemotherapy (NAC) is critical for making appropriate treatment decisions.
Previously, numerous studies have shown that clinicopathological indicators such as estrogen receptor (ER), progesterone receptor (PgR), KI67, and tumor infiltrating lymphocytes (TILs) independently predict pCR in patients receiving NAC containing anti-targeted drugs (9,10). Currently, immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) have been used to determine HER-2 expression levels in breast cancer tissues in clinical trials. According to the 2018 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines, all patients diagnosed with breast cancer should undergo HER-2 testing to formulate appropriate treatment strategies for achieving better treatment outcomes (11). Our previous study showed that patients with HER-2-positive (IHC 3+) breast cancer have significantly higher pCR rates, which indicates that the HER-2 protein expression level might serve as an independent predictor of the NAC (12). Some studies have also shown that the level of the HER-2/CEP17 (chromosome enumeration probe 17) ratio correlates with pCR rates in patients with HER-2-positive breast cancer after treatment with NAC plus trastuzumab (13-15). However, dual HER-2 blocking therapy is not effective in all patients with HER-2-amplified breast cancer. Meanwhile, few studies have investigated the relationship between the level of HER-2/CEP17 ratio and the pCR of NAC regimens containing trastuzumab and pertuzumab, as dual-target therapy has become the standard neoadjuvant regimen for patients with HER2-positive early breast cancer. Therefore, we used FISH to analyze the relationship between the HER-2/CEP17 ratio levels and pCR in response to HER-2 dual-blocking drugs (pertuzumab and trastuzumab). In the present study, the HER-2/CEP17 ratio was used as a molecular biological index to evaluate its correlation and predictive effect on complete pathological remission after NAC for breast cancer. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-632/rc).
Methods
Patients
To address the goal, the investigators designed a retrospective cohort study. From January 1, 2020 to May 30, 2021, patients with histologically confirmed HER-2-positive early or locally advanced breast cancer who underwent NAC followed by surgery at The Affiliated Cancer Hospital of Zhengzhou University were included in the study. Data regarding demography, pathology, ER, PgR, Ki-67 index, and pCR of enrolled patients was extracted and analyzed.
The following criteria were used for patient selection: (I) age ranging from 18 to 70 years, an Eastern Cooperative Oncology Group score of 0–1, and a baseline left ventricular ejection fraction of ≥55%; (II) confirmation of invasive breast cancer by ultrasound-guided core needle biopsy before chemotherapy; (III) IHC results suggesting HER-2 (IHC 2+) and FISH results showing its amplification to meet HER-2 positive requirements; (IV) no distant metastases detected by imaging examination; (V) receiving neoadjuvant regimens, including trastuzumab and pertuzumab targeted therapy; (VI) no history of chemotherapy, endocrine therapy, radiotherapy, or other anticancer treatments; (VII) breast-conserving surgery or modified radical mastectomy for breast cancer was completed after 6 cycles of NAC with dual-targeted therapy; and (VIII) complete clinicopathological data and treatment data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Henan Cancer Hospital (No. 2017407). Individual consent for this retrospective analysis was waived.
IHC and FISH
Formalin-fixed, paraffin-embedded surgical tissue samples were cut into 4-µm-thick continuous slices for IHC. The samples were positive for ER and PgR based on positive staining for 1% or more of the nuclei. Simultaneous negative expression of ER and PgR was defined as hormone receptor (HR) negative, while positive expression for at least 1 receptor was defined as HR positive. The Ki-67 index was categorized as high expression (≥30%) and low expression (<30%), with a cut-off value of 30%.
According to the updated 2018 ASCO/CAP Clinical Practice Guidelines (11), each patient was diagnosed as HER-2 positive based on the following criteria: IHC 0 and IHC 1+ were defined as HER-2 negative, IHC 2+ was categorized as HER-2 borderline, and IHC 3+ was categorized as HER-2 positive. The dual-probe FISH assay was conducted routinely on all tumor specimens with HER-2 (IHC 2+) for final HER-2 classification. Tumors were considered HER-2 positive if dual-probe HER-2/CEP17 ratio was >2 or ratio <2, but with HER-2 copy number >6 signals per cell.
Neoadjuvant treatment regimen
All patients received a 6-cycle neoadjuvant regimen comprising a dual-targeted therapy that included trastuzumab at the loading dose of 8 mg/kg, followed by a 6 mg/kg dose and pertuzumab at the loading dose of 840 mg/kg, followed by a 420 mg/kg dose; both drugs were administered once every 3 weeks. The majority of patients received TCbHP regimen (containing paclitaxel 75 mg/m2, day 1 plus carboplatin, area under the curve=6, day 1, once every 3 weeks) and PHP regimen (containing albumin paclitaxel 125 mg/m2, days 1/8/15, once every 3 weeks). A small number of patients received the NAC regimen THP containing docetaxel (80–100 mg/m2) once every 3 weeks. All patients eventually underwent surgery after 6 cycles of NAC. The surgical procedure was chosen by the surgeon according to the specific condition of the patient. The pCR was described as the absence of histological evidence of malignancy in both primary and metastatic lymph nodes of patients with breast cancer postoperatively or the presence of only carcinoma in situ components (ypT0/isN0).
Statistical analysis
The primary outcome variable was pCR. The primary predictive variable was the HER-2/FISH ratio treated as a dichotomized variable with a cut-off value of 6, as determined from a previous report (13). Other variables included age (≤50 vs. >50 years), menopausal status, tumor-node-metastasis (TNM) stage, ER, PgR, and Ki-67 index. Association between clinicopathologic variables and pCR was firstly analyzed using χ2-test or Fisher’s exact probability method, the significant variables were then evaluated in logistic regression model to determine the independence. Spearman’s rank correlation test was used to analyze the correlation between different HER-2/CEP17 ratios and pCR rates. All statistical analyses were performed using Statistical Package for the Social Sciences 20.0. Statistical significance was set at two-sided P<0.05.
Results
Patient and tumor characteristics
Sixty-nine women with HER2 IHC 2+/FISH-negative breast cancer were included in this study, and their clinicopathological characteristics and treatment details are provided in Table 1. The mean age was 51.78 years (25–67 years). Of these patients, 35 (50.7%) were premenopausal. Clinical stages at diagnosis were Stage II for 31 patients (44.9%) and Stage III for 38 patients (55.1%). Among all patients, 40.6% (28/69) were ER positive, 52.2% (36/69) were PgR positive, and 75.4% (52/69) had a Ki-67 index of ≥30%.
Table 1
| Characteristic | N (n=69) | % |
|---|---|---|
| Age (years) | ||
| ≤50 | 31 | 44.9 |
| >50 | 38 | 55.1 |
| Menopausal status | ||
| Postmenopausal | 34 | 49.3 |
| Premenopausal | 35 | 50.7 |
| Clinical tumor staging | ||
| cT1 | 2 | 2.9 |
| cT2 | 52 | 75.4 |
| cT3 | 10 | 14.5 |
| cT4 | 5 | 7.2 |
| Clinical lymph node staging | ||
| cN0 | 19 | 27.5 |
| cN1 | 16 | 23.2 |
| cN2 | 17 | 24.6 |
| cN3 | 17 | 24.6 |
| AJCC stage* | ||
| IIA | 17 | 24.6 |
| IIB | 14 | 20.3 |
| IIIA | 15 | 21.7 |
| IIIB | 5 | 7.2 |
| IIIC | 18 | 26.1 |
| ER status | ||
| Positive | 28 | 40.6 |
| Negative | 41 | 59.4 |
| PgR status | ||
| Positive | 36 | 52.2 |
| Negative | 33 | 47.8 |
| Ki67 index | ||
| ≥30% | 52 | 75.4 |
| <30% | 17 | 24.6 |
| HER-2/CEP17 ratio | ||
| High level (≥6) | 20 | 29.0 |
| Low level (<6) | 49 | 71.0 |
| Chemotherapy regimens | ||
| TCbHP | 57 | 82.6 |
| PHP | 8 | 11.6 |
| THP | 4 | 5.8 |
| RECIST-based treatment response | ||
| CR | 26 | 37.7 |
| PR | 39 | 56.5 |
| SD | 4 | 5.8 |
| Pathologic response of the primary tumor | ||
| pCR (T0/is) | 30 | 43.5 |
| Non-pCR | 39 | 56.5 |
| Pathologic response of the primary tumor and lymph node | ||
| pCR | 22 | 31.9 |
| Non-pCR | 47 | 68.1 |
*, according to AJCC Cancer Staging Manual, Eighth Edition (2019). AJCC, American Joint Committee on Cancer; ER, estrogen receptor; PgR, progesterone receptor; HER-2, human epidermal growth factor receptor-2; CEP17, chromosome enumeration probe 17; TCbHP, docetaxel, carboplatin, trastuzumab, and pertuzumab; PHP, albumin paclitaxel, trastuzumab, and pertuzumab; THP, docetaxel, trastuzumab, and pertuzumab; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; pCR, pathological complete response.
Association between clinicopathological parameters and the pCR rate
In this study, postsurgical pathology showed that 22 patients (31.9%) achieved pCR, while 47 patients (68.1%) did not. The results of the χ2-test between the pCR rate and the clinicopathological parameters are shown in Table 2. The pCR rate of patients with a high level of HER-2/CEP17 ratio (≥6) was 55% compared with 22.4% in patients with low levels of HER-2/CEP17 ratio (<6) (P=0.008). In the subgroup analysis, the pCR rates were 19.5% and 50% in the ER-negative and ER-positive patient groups, respectively. The pCR rates of lymph node-negative and lymph node-positive patients were 63.2% and 20%, respectively. The univariate analyses revealed that pCR was prominently interrelated with clinical tumor staging (P=0.040), clinical lymph node staging (P=0.001), American Joint Committee on Cancer TNM staging (P=0.008), and Ki-67 index (P=0.080) (Table 2).
Table 2
| Characteristics | Pathological evaluation, n (%) | χ2 | P value | |
|---|---|---|---|---|
| pCR (n=22) | Non-pCR (n=47) | |||
| Age (years) | 0.004 | 0.952 | ||
| ≤50 | 10 (32.3) | 21 (67.7) | ||
| >50 | 12 (31.6) | 26 (68.4) | 0.189 | 0.664 |
| Menopausal status | ||||
| Postmenopausal | 10 (29.4) | 24 (70.6) | ||
| Premenopausal | 12 (34.3) | 23 (65.7) | ||
| Clinical tumor staging | 4.226 | 0.040 | ||
| cT1–2 | 21 (38.9) | 33 (61.1) | ||
| cT3–4 | 1 (6.7) | 14 (93.3) | ||
| Clinical lymph node staging | 11.808 | 0.001 | ||
| cN0 | 12 (63.2) | 7 (36.8) | ||
| cN1–3 | 10 (20.0) | 40 (80.0) | ||
| TNM AJCC stage* | 7.059 | 0.008 | ||
| II | 15 (48.4) | 16 (51.6) | ||
| III | 7 (18.4) | 31 (81.6) | ||
| ER status | 7.121 | 0.010 | ||
| Positive | 14 (50.0) | 14 (50.0) | ||
| Negative | 8 (19.5) | 33 (80.5) | ||
| PgR status | 1.701 | 0.192 | ||
| Positive | 14 (38.9) | 22 (61.1) | ||
| Negative | 8 (24.2) | 25 (75.8) | ||
| Ki67 index | 3.065 | 0.080 | ||
| ≥30% | 20 (38.5) | 32 (61.5) | ||
| <30% | 2 (11.8) | 15 (88.2) | ||
| HER-2/CEP17 ratio | 6.929 | 0.008 | ||
| High level (≥6.0) | 11 (55.0) | 9 (45.0) | ||
| Low level (<6.0) | 11 (22.4) | 38 (77.6) | ||
*, according to AJCC Cancer Staging Manual, Eighth Edition (2019). pCR, pathological complete response; TNM, tumor-node-metastasis; AJCC, American Joint Committee on Cancer; ER, estrogen receptor; PgR, progesterone receptor; HER-2, human epidermal growth factor receptor-2; CEP17, chromosome enumeration probe 17.
Predictive value of the HER-2/CEP17 ratio regarding pCR
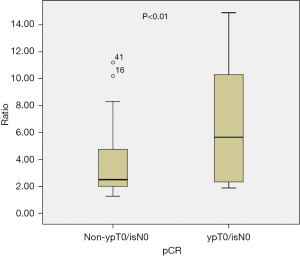
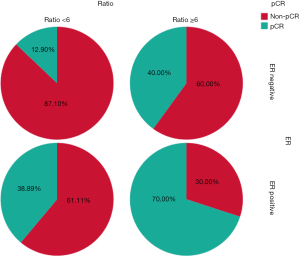
Overall, NAC based on the dual anti-HER-2 therapy had a pCR rate of 31.9% in ypT0/isN0 and 43.5% in ypT0/is. Patients who responded to trastuzumab-based neoadjuvant treatment had a median ratio of 5.65 (range, 1.88–14.90), whereas those who did not respond had a median ratio of 2.5 (range, 1.27–11.20, P<0.01) (Figure 1). According to the cut-off value of 6, patients were categorized into the low HER-2/CEP17 ratio group (<6) and the high HER-2/CEP17 ratio group (≥6). Patients in the high HER-2/CEP17 ratio group achieved pCR in 11 of 20 cases (55%), which was significantly higher than those in the low HER-2/CEP17 ratio group (11/49, 22.4%, P=0.008) (Table 2). Interestingly, 7 of 10 (70.0%) ER-positive tumors with a HER2/CEP17 ratio ≥6 achieved pCR, while 7 of 18 (38.89%) with a low ratio (<6) achieved pCR. We also observed that within the ER-negative tumors, 4 of 10 (40.0%) cancers with HER2/CEP17 ratio ≥6 achieved pCR, while only 4 of 31 (12.9%) tumors with a low ratio had a pCR (Figure 2). In the multivariate logistic regression analyses, a significant improvement in the pCR rate of tumors was found in the high HER-2/CEP17 ratio group [odds ratio (OR): 5.203, 95% confidence interval (CI): 1.302–20.783, P=0.02].
Finally, 4 factors with P<0.05 were analyzed by the multivariate analysis. Patients with a higher HER-2/CEP17 ratio (≥6) were more likely to reach pCR after NAC, while clinical lymph node staging of N1–3 were less likely to reach pCR (OR: 0.099; 95% CI: 0.023–0.430, P=0.002). In contrast, patients with ER-negative breast cancer were less likely to reach pCR after NAC. Therefore, these factors were independent positive prognostic factors (Table 3).
Table 3
| Variables | OR | 95% Cl | P value |
|---|---|---|---|
| ER status | |||
| Negative | 1 | ||
| Positive | 4.036 | (1.094–14.890) | 0.036 |
| Ki67 index | |||
| <30% | 1 | ||
| ≥30% | 3.439 | (0.611–19.346) | 0.161 |
| Clinical lymph node staging | |||
| cN0 | 1 | ||
| cN1–3 | 0.099 | (0.023–0.430) | 0.002 |
| HER-2/CEP17 ratio | |||
| Low level | 1 | ||
| High level | 5.203 | (1.302–20.783) | 0.020 |
pCR, pathological complete response; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; OR, odds ratio; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor-2; CEP17, chromosome enumeration probe 17.
Discussion
Breast cancer is a heterogeneous disease with various phenotypes, and approximately 20–25% of invasive breast cancers show amplification or overexpression of the HER-2 gene (16). Both early and metastatic breast cancers that meet the HER-2-positive criteria should be treated with regimens containing anti-HER-2 drugs. Preoperative neoadjuvant therapy with anti-HER-2 drugs in patients with locally advanced HER-2-positive breast cancer not only reduces the size of primary and axillary lymph node metastases, which provides patients with surgical opportunities to improve prognosis, but also identifies patients who could benefit from treatment or those who have poor responses (8,17). By using the HER-2-positive breast cancer treatment drug trastuzumab, and its combination with other drugs, the prognosis of patients with HER-2-positive breast cancer has greatly improved (18). Therefore, timely and accurate detection and evaluation of breast cancer treatment options and prognosis assessment are extremely important, especially for the development of individualized anti-HER-2-positive targeted therapeutic regimens and the evaluation of drug efficacy. In the present study, we assessed the effect of the HER-2/CEP17 ratio detected by FISH on the pCR rate in IHC 2+/FISH-amplified breast cancers treated with the NAC combination of trastuzumab and pertuzumab. We found that, among the 69 IHC 2+/FISH-amplified breast cancers, tumors with a HER-2/CEP17 ratio of ≥6 showed a higher tendency to achieve pCR compared with tumors with a HER-2/CEP17 ratio of <6.
Several studies have confirmed that a high HER-2/CEP17 ratio is associated with a high pCR rate in HER-2-positive breast cancer after NAC. Singer et al. studied 114 patients with HER-2-positive breast cancer who received neoadjuvant anti-HER-2-targeted therapy and chemotherapy (13). The study pooled and analyzed the relationship between HER-2 gene amplification and post-NAC pCR rate in the enrolled patients. The median HER-2/CEP17 ratio in patients who achieved pCR in that study was 7 compared with 3.82 for patients who did not achieve pCR when defined as ypT0/isN0. The pCR rates were higher in patients with a HER-2/CEP17 ratio of ≥6 compared with patients with a HER-2/CEP17 ratio of <6 (82.8% vs. 38.3%, P<0.001). Patients with a HER-2/CEP17 ratio of >6 were more likely to achieve pCR after NAC than those with a HER-2/CEP17 ratio of <6 (OR: 5.08, 95% CI: 1.86–13.90, P=0.002). Arnould et al. summarized the amplification of the HER-2 gene and postoperative pathology in 93 patients with HER-2-positive breast cancer who received neoadjuvant anti-HER2-targeted therapy and chemotherapy, and found that the pCR rate after NAC was higher than that in patients with low amplification (56% vs. 22%, P<0.005) (14). Greenwell et al.’s findings also supported those results. In their study, Greenwell et al. pooled and analyzed the HER-2/CEP17 ratio to postoperative pathology in 10,181 patients with HER-2-positive breast cancer who received NAC in the National Cancer Database (15). The results showed that the HER-2/CEP17 ratio was significantly higher in patients who achieved pCR after NAC (P<0.001). The pCR rate also increased with an increase in the HER-2/CEP17 ratio. In accordance with the present results, a previous study also demonstrated that HER-2/CEP17 ratios were relatively high in patients with HER-2/CEP17-positive breast cancer receiving neoadjuvant therapy with trastuzumab; this was associated with a significantly higher probability of achieving pCR by fixing the cut-off ratio at 6 to differentiate between a high-level ratio and low-level ratio, regardless of the absence or presence of ductal carcinoma in situ (DCIS) (13). In the present study, 55% of patients with HER-2/CEP17 ratios ≥6 achieved pCR, which was significantly higher than the 22.4% of patients with HER-2/CEP17 ratios <6 who achieved pCR.
We also demonstrated that patients with higher HER-2/CEP17 ratios in HER-2-positive breast tumors showed a significantly higher probability of pCR after combined neoadjuvant treatment with trastuzumab and pertuzumab, which was in line with the findings of previously published studies (19). In the present study, we found that pCR (defined by ypT0/isN0) occurred in only 22 of 69 patients (31.9%) when pertuzumab + NAC based on trastuzumab was used, and even with the relatively non-restrictive definition of pCR (ypT0/isN0), only 43.5% (30/69) of patients achieved pCR. This value was relatively lower than the pCR rate reported in the NeoALTTO study (51.3%) and Train-2 study (68%) (20,21). Several factors could explain this difference. First, all patients included in the present study had IHC 2+/FISH-amplified breast cancers, which were confirmed to be less likely to achieve pCR than IHC 3+ breast cancers investigated in our previous study (19.3% vs. 31.4%) (12). Second, our study included a relatively large number of locally advanced Stage III patients (38/69, 55.1%) and patients with N3 stage tumors (24.6%, 17/69) and T3–4 stage tumors (21.7%, 15/69). Moreover, in our current study, the ER-positive status was a predictor of pCR in IHC 2+/FISH-amplified breast cancers, which was in contrast with the findings of most clinical studies to the best of our knowledge. This result could be explained by the fact that 10 of the 28 ER-positive patients had a high HER-2/CEP17 ratio, while only 10 of the 41 ER-negative patients had a high HER-2/CEP17 ratio, which could be due to the limited number of cases.
In the present study, we analyzed the HER-2/CEP17 ratio in IHC 2+/FISH-amplified breast cancers treated with neoadjuvant therapy containing pertuzumab and trastuzumab. Our findings indicated that a HER-2/CEP17 ratio of ≥6 is significantly more likely to achieve pCR. Recently, the KATHERINE study showed that, among patients with HER-2-positive early breast cancer without pCR after neoadjuvant therapy, adjuvant T-DM1 therapy was associated with a 50% lower risk of invasive breast cancer recurrence than trastuzumab therapy alone (7). The widespread application of T-DM1 in neoadjuvant therapy in the future can possibly further improve the pCR rate of HER-2-positive patients with high HER-2/CEP17 ratios and even provide survival benefits. Furthermore, it would be of great interest to investigate in larger studies whether higher pCR rates or survival benefits could be obtained by adding a new generation of targeted drugs, such as T-DM1 and DS8201, to pertuzumab and trastuzumab-based NAC (17).
To the best our knowledge, our real-world research based on the level of the HER-2/CEP17 ratio to predict the pCR of patients with HER-2-positive breast cancer following NAC is the first study to focus on IHC 2+/FISH-amplified breast cancer treated with neoadjuvant pertuzumab and trastuzumab therapy. The present study, however, has several limitations. First, because of the limited number of specific cases in this study, the influence of different regions, different age groups, and different races on the outcome could not be determined. Second, the relevant data require a large number of long-term, multicenter investigations and evaluation of clinical efficacy so that the application and clinical potential of the targeted drugs can be evaluated more effectively and accurately (21,22). Third, the sample size was not calculated before the study, there might be some undetected predictors, and more researches were needed.
Conclusions
A HER-2/CEP17 ratio ≥6.0 might be related to more achievement of pCR in the neoadjuvant anti-HER-2 dual-targeted therapies. Further studies are needed to validate the finding.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-632/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-632/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-632/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Henan Cancer Hospital (No. 2017407). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [Crossref] [PubMed]
- Piccart M, Procter M, Fumagalli D, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years' Follow-Up. J Clin Oncol 2021;39:1448-57. [Crossref] [PubMed]
- Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 2005;23:3676-85. [Crossref] [PubMed]
- Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2018;19:115-26. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Meisel JL, Zhao J, Suo A, et al. Clinicopathologic Factors Associated With Response to Neoadjuvant Anti-HER2-Directed Chemotherapy in HER2-Positive Breast Cancer. Clin Breast Cancer 2020;20:19-24. [Crossref] [PubMed]
- Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983-91. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-22. [Crossref] [PubMed]
- Yan H, Xiao H, Zhu J, et al. Association Between the HER2 Protein Expression Level and the Efficacy of Neoadjuvant Chemotherapy in HER2-Positive Breast Cancer. Cancer Manag Res 2020;12:12715-22. [Crossref] [PubMed]
- Singer CF, Tan YY, Fitzal F, et al. Pathological Complete Response to Neoadjuvant Trastuzumab Is Dependent on HER2/CEP17 Ratio in HER2-Amplified Early Breast Cancer. Clin Cancer Res 2017;23:3676-83. [Crossref] [PubMed]
- Arnould L, Arveux P, Couturier J, et al. Pathologic complete response to trastuzumab-based neoadjuvant therapy is related to the level of HER-2 amplification. Clin Cancer Res 2007;13:6404-9. [Crossref] [PubMed]
- Greenwell K, Hussain L, Lee D, et al. Complete pathologic response rate to neoadjuvant chemotherapy increases with increasing HER2/CEP17 ratio in HER2 overexpressing breast cancer: analysis of the National Cancer Database (NCDB). Breast Cancer Res Treat 2020;181:249-54. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020;382:610-21. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Li F, Ju Q, Gao C, et al. Association of HER-2/CEP17 Ratio and HER-2 Copy Number With pCR Rate in HER-2-Positive Breast Cancer After Dual-Target Neoadjuvant Therapy With Trastuzumab and Pertuzumab. Front Oncol 2022;12:819818. [Crossref] [PubMed]
- de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 2014;15:1137-46. [Crossref] [PubMed]
- van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018;19:1630-40. [Crossref] [PubMed]
- Xuan Q, Ji H, Tao X, et al. Quantitative assessment of HER2 amplification in HER2-positive breast cancer: its association with clinical outcomes. Breast Cancer Res Treat 2015;150:581-8. [Crossref] [PubMed]


