
The usefulness of red blood cell distribution width and its ratio with platelet count in breast cancer after surgery and adjuvant treatment: a retrospective study
Highlight box
Key findings
• Elevated red blood cell distribution width (RDW) and RDW-to-platelet count to ratio (RPR) levels after surgery and adjuvant therapy are associated with poor disease-free survival and overall survival in patients with breast cancer.
What is known and what is new?
• When the RDW and RPR groups had lower levels before treatment and then increased to a higher level after treatment, the prognostic value was more significantly associated with poor survival.
What is the implication, and what should change now?
• As RDW and RPR are simple and cost-effective, they are useful biomarkers for improved risk assessment in breast cancer.
Introduction
Breast cancer is one of the most commonly diagnosed cancers worldwide and is one of the most frequent causes of cancer-related deaths (1). Furthermore, in breast cancer, local relapse and distant metastasis are important for clinical management. In breast cancer patients, assessment of the patient’s prognosis is very important because of clinical decision-making in care and treatment after surgery. Several molecular diagnostic tests have been applied to obtain reliable prognostic information, such as Oncotype Dx. However, its clinical use is still limited owing to its uncertain role and high cost (2). Therefore, an indicator that is simple, convenient, and inexpensive to administer should be helpful for early recurrence detection and for guiding treatment decisions.
Recently, red blood cell distribution width (RDW) has been investigated for its association with cancer (3). RDW is a widely used laboratory parameter reported in complete blood count tests, and it reflects the heterogeneity in the size of circulating erythrocytes (4). Higher RDW values indicate greater variation in the size of red blood cells. Platelets play an important role in cancer progression and metastasis (5,6). Elevated platelets are associated with poor prognosis in cancers, such as colorectal cancer, endometrial cancer, gastric cancer, ovarian cancer, and pancreatic cancer (7-11). Furthermore, the RDW-to-platelet count to ratio (RPR) reflects the severity of inflammation and is used to predict fibrosis in chronic hepatitis (12). Furthermore, RPR is increasingly being recognized to have an important role and a potential biomarker in breast cancer patients (13). However, there are no reports on the prognostic value of RPR in breast cancer before and after treatment. Therefore, in this study, we aimed to investigate the prognostic value of RDW and RPR in breast cancer before and after treatment. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-410/rc).
Methods
Patients selection and data extraction
We retrospectively reviewed 411 patients diagnosed with primary breast cancer at Gyeongsang National University Changwon Hospital between December 2009 and December 2015. The exclusion criteria were as follows: age <19 years; noninvasive breast cancer or stage IV breast cancer; preoperative treatment such as neoadjuvant chemotherapy; insufficient information on laboratory data or pathologic results; and other diseases such as hematological disorders, liver cirrhosis, and heart failure. Ultimately, 395 patients with breast cancer were enrolled and analyzed in this study. All information, such as pathological results and, laboratory data, was obtained from medical records. The baseline characteristics of the enrolled patients are summarized in Table 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Review Board of the Gyeongsang National University Changwon Hospital (No. GNUCH 2021-11-013) and the requirement for informed consent was waived because it was a retrospective study.
Table 1
| Factors | Total number =395, n (%) |
|---|---|
| Age (years) | |
| <48.5 | 145 (36.7) |
| ≥48.5 | 250 (63.3) |
| Histology | |
| IDC | 338 (85.6) |
| ILC | 16 (4.1) |
| Mucinous | 12 (3.0) |
| Medullary | 6 (1.5) |
| Other | 23 (5.8) |
| Surgery | |
| Mastectomy | 156 (39.5) |
| BCS | 236 (59.7) |
| Others | 3 (0.8) |
| Tumor size | |
| ≤2.0 cm | 249 (63.0) |
| >2.0 cm | 146 (27.0) |
| Node | |
| N0 | 251 (63.5) |
| N1–3 | 144 (36.5) |
| HG | |
| 1–2 | 238 (60.2) |
| 3 | 127 (32.2) |
| Unknown | 30 (7.6) |
| ER status | |
| Negative | 131 (33.2) |
| Positive | 264 (66.8) |
| PR status | |
| Negative | 176 (44.6) |
| Positive | 219 (55.4) |
| HER2 status | |
| Negative | 312 (79.0) |
| Positive | 83 (21.0) |
| Subtype | |
| Luminal A | 251 (63.5) |
| Luminal B | 35 (8.9) |
| HER2 | 38 (9.6) |
| Triple negative | 71 (18.0) |
| LV invasion | |
| Negative | 339 (85.5) |
| Positive | 56 (14.5) |
| Recurrence | |
| No | 347 (87.8) |
| Yes | 48 (12.2) |
| Death | |
| No | 381 (96.5) |
| Yes | 14 (3.5) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; BCS, breast-conserving surgery; HG, histologic grade; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; LV, lymphovascular.
Clinical assessment
All patients underwent surgical resection including breast-conserving surgery or total mastectomy. Subsequently, adjuvant treatment, such as chemotherapy, radiotherapy, and endocrine therapy, was administered. After surgery, all patient received adjuvant chemotherapy followed by four cycles of doxorubicin (60 mg/m2)/cyclophosphamide (600 mg/m2) chemotherapy every 21 days and received four cycles of docetaxel (75 mg/m2) chemotherapy every 21 days in patient who have metastatic axillary lymph node or high-risk patients. Radiotherapy following breast-conserving surgery and total mastectomy in high-risk patients. And estrogen-receptor positive patient started endocrine therapy as like tamoxifen (in pre-menopausal woman) or aromatase-inhibitor (in post-menopausal woman). A regular follow-up evaluation was performed at 6-month intervals for during 1–5 years and at 1-year intervals for during 5–10 years after treatment. Follow-up investigations included physical examinations, laboratory analysis (complete blood count and carbohydrate-anti-gene 15-3 levels), and radiological assessment (mammography, ultrasonography, and bone scan).
Biochemical measurements
Venous blood samples were obtained via peripheral venous puncture before treatment. After treatment, venous blood samples were obtained after surgery, adjuvant chemotherapy, and radiotherapy, before and after the median time of blood sampling was 29 months. RDW and platelet counts were measured using a hematology analyzer (XN-10; Sysmex Corporation, Kobe, Japan). According to the manufacturer’s instructions, the normal range of RDW is 11.5–14.5%, and platelet is 13–40 (×104/µL). The RPR was calculated by dividing the RDW by the platelet count (×104/µL).
Statistical analysis
Statistical analysis was performed using the SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). The distribution of RDW and RPR are in Figure 1. The optimal cut-off values for RDW and PRP were determined by receiver operating characteristic (ROC) curve analysis by identifying the highest Youden index (sensitivity + specificity − 1) before and after treatment. As shown in Figure 2A and Table 2 for DFS, the area under the ROC curve (AUC) for pre-RDW, post-RDW, pre-RPR and post-RPR was 0.474 (P=0.872), 0.697 (P<0.000), 0.481 (P=0.718), and 0.592 (P=0.081), respectively. ROC analysis based on RDW, RPR for OS, as shown in Figure 2B and Table 3, the area under the ROC curve (AUC) for pre-RDW, post-RDW, pre-RPR and post-RPR was 0.457 (P=0.729), 0.736 (P=0.009), 0.486 (P=0.686), and 0.693 (P=0.037), respectively.
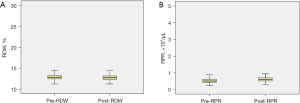
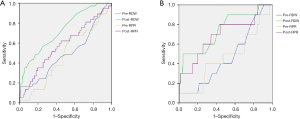
Table 2
| Characteristics | Cut-off value | Specificity | Sensitivity | AUC (95% CI) | Treatment |
|---|---|---|---|---|---|
| RDW | 12.75 | 0.444 | 0.478 | 0.474 (0.383–0.566) | Before |
| RDW | 12.85 | 0.571 | 0.625 | 0.697 (0.613–0.780) | After |
| RPR | 0.517 | 0.524 | 0.50 | 0.481 (0.394–0.569) | Before |
| RPR | 0.631 | 0.590 | 0.579 | 0.592 (0.490–0.694) | After |
RDW, red blood cell distribution width; RPR, red blood cell distribution width to platelet count ratio; DFS, disease-free survival; AUC, area under the curve; CI, confidence interval.
Table 3
| Characteristics | Cut-off value | Specificity | Sensitivity | AUC (95% CI) | Treatment |
|---|---|---|---|---|---|
| RDW | 12.75 | 0.449 | 0.429 | 0.457 (0.304–0.610) | Before |
| RDW | 13.05 | 0.655 | 0.643 | 0.736 (0.579–0.892) | After |
| RPR | 0.510 | 0.501 | 0.500 | 0.486 (0.319–0.653) | Before |
| RPR | 0.660 | 0.656 | 0.600 | 0.693 (0.508–0.879) | After |
RDW, red blood cell distribution width; RPR, red blood cell distribution width to platelet count ratio; OS, overall survival; AUC, area under the curve; CI, confidence interval.
Survival curves were estimated using the high RDW group (≥ cutoff value)/high RPR group (≥ cutoff value) and low RDW group (< cutoff value)/RPR group (< cutoff value) before and after treatment. Kaplan-Meier curve analysis and log-rank tests were used to compare patient survival rate. Independent prognostic factors were identified via univariate analysis using the Cox proportional hazards model to identify the independent variables associated with survival. Hazard ratios (HRs) estimated using Cox regression were reported as relative risks with corresponding 95% confidence intervals (CIs). Multivariate Cox regression analysis was performed for parameters found to be significant in the univariate analysis. Statistically significant was set at P value <0.05.
Results
Patient’s characteristics
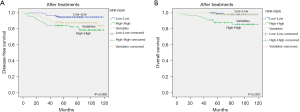
A total of 395 patients with breast cancer enrolled in this study. The median age was 48.5 years old (range, 24–89 years). The median follow-up period was 84 months. After follow-up, 48 patients (12.2%) experienced recurrence and death of patients were 14 (3.5%) died. The study population was divided into four groups (high RDW, low RDW, high PRP, and low PRP) after treatment according to the optimized cut-off values determined by ROC analysis (Figure 3). The optimal cutoff values for disease-free survival (DFS) and, overall survival (OS) before and after treatment are shown in Tables 2,3.

Survival
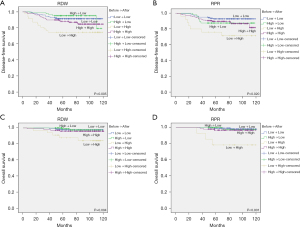
There was no significant difference in DFS and OS between the high and low RDW groups before treatments. Likewise, no DFS or OS differences were found between the high and low RPR groups in before treatment. However, after treatment, the univariate analysis showed that the DFS and OS rates of the high RDW group were significantly lower than those of low RDW group (P=0.015 and P=0.016, respectively; Figure 3A,3B). In addition, multivariate Cox regression analysis indicated that elevated RDW was positively associated with DFS (HR =2.067; 95% CI: 1.085–3.937; P=0.027) and OS (HR =3.272; 95% CI: 0.832–12.866; P=0.05) (Tables 4,5). Although not P<0.05, the univariate analysis showed that the DFS and OS rates in the high RPR group were significantly lower than those in the low RPR group after treatment (P=0.053 and P=0.043, respectively; Figure 3C,3D). We analyzed two groups: a high RDW/high RPR group that satisfied both the high RDW and high RPR groups and a low RDW/low RPR group that satisfied both the low RDW and low RPR groups. As shown in Figure 4, patients with high RDW/high RPR had significantly lower DFS and OS rates than those with low RDW/low RPR (P=0.008 and P<0.001, respectively). Furthermore, Cox regression multivariate analysis revealed that high RDW/high RPR was associated with poor DFS (HR =10.467; 95% CI: 4.863–22.527; P<0.001) and OS (HR =30.461; 95% CI: 5.138–180.575; P<0.001) (Tables 4,5). Finally, we examined the effects of RDW and RPR changes after the initial treatment between the lower and higher group. Kaplan-Meier analysis revealed that patients with changed value of lower RDW and RPR before treatment and higher RDW and RPR after treatment had significantly poorer DFS (P=0.005 and P=0.02, respectively) and OS (P=0.004 and P<0.001, respectively) (Figure 5). In addition, Cox regression multivariable analysis showed that changing the lower group to the higher group was associated with poor DFS [HR(RDW) =4.947; 95% CI: 2.068–11.834; P<0.001 and HR(RPR) =6.548; 95% CI: 2.360–18.168; P<0.001] and OS [HR(RDW) =10.119; 95% CI: 1.853–55.249; P=0.008 and HR(RPR) =132.6; 95% CI: 3.689–4,767.341; P=0.007] (Tables 4,5).
Table 4
| Characteristic | HR | 95% CI | P value |
|---|---|---|---|
| Age (years) | |||
| <50 | 1 | ||
| ≥50 | 1.261 | 0.695–2.286 | 0.444 |
| T stage | |||
| T1 | 1 | ||
| T2 | 1.260 | 0.651–2.438 | 0.493 |
| T3 | 2.016 | 0.703–5.778 | 0.192 |
| N stage | |||
| N0 | 1 | ||
| N1 | 2.530 | 1.068–5.993 | 0.035 |
| N2 | 6.266 | 2.413–16.274 | <0.001 |
| N3 | 8.642 | 3.391–22.028 | <0.001 |
| LV invasion | |||
| Negative | 1 | ||
| Positive | 7.905 | 0.745–83.870 | 0.086 |
| Subtype | |||
| Luminal A | 1 | ||
| Luminal B | 1.823 | 0.734–4.530 | 0.196 |
| HER2 | 2.741 | 1.131–6.646 | 0.026 |
| Triple negative | 1.459 | 0.570–3.739 | 0.431 |
| RDW after treatment | |||
| < COV | 1 | ||
| ≥ COV | 2.067 | 1.085–3.937 | 0.027 |
| RPR after treatment | |||
| < COV | 1 | ||
| ≥ COV | 2.233 | 1.073–4.649 | 0.032 |
| RDW & RPR after treatment | |||
| Low-low | 1 | ||
| High-high | 10.467 | 4.863–22.527 | <0.001 |
| RDW before to after treatment | |||
| Low to low | 1 | ||
| Low to high | 4.947 | 2.068–11.834 | <0.001 |
| RPR before to after treatment | |||
| Low to low | 1 | ||
| Low to high | 6.548 | 2.360–18.168 | <0.001 |
HR, hazard ratio; CI, confidence interval; LV, lymphovascular; HER2, human epidermal growth factor receptor 2; RDW, red blood cell distribution width; COV, cut-off value; RPR, red blood cell distribution width to platelet count ratio.
Table 5
| Characteristic | HR | 95% CI | P value |
|---|---|---|---|
| Age (years) | |||
| <50 | 1 | ||
| ≥50 | 0.870 | 0.290–2.607 | 0.803 |
| T stage | |||
| T1 | 1 | ||
| T2 | 0.216 | 0.042–1.100 | 0.065 |
| T3 | 1.422 | 0.150–13.498 | 0.759 |
| N stage | |||
| N0 | 1 | ||
| N1 | 3.546 | 0.572–21.967 | 0.064 |
| N2 | 30.055 | 3.446–262.099 | 0.002 |
| N3 | 15.530 | 2.792–86.387 | 0.002 |
| LV invasion | |||
| Negative | 1 | ||
| Positive | 13.092 | 0.401–426.990 | 0.148 |
| Subtype | |||
| Luminal A | 1 | ||
| Luminal B | – | – | – |
| HER2 | 20.379 | 3.557–116.751 | 0.001 |
| Triple negative | 5.076 | 0.738–34.898 | 0.099 |
| RDW after treatment | |||
| < COV | 1 | ||
| ≥ COV | 3.272 | 0.832–12.866 | 0.05 |
| RPR after treatment | |||
| < COV | 1 | ||
| ≥ COV | 2.510 | 0.407–15.489 | 0.322 |
| RDW & RPR after treatment | |||
| Low-low | 1 | ||
| High-high | 30.461 | 5.138–180.575 | <0.001 |
| RDW before to after treatment | |||
| Low to low | 1 | ||
| Low to high | 10.119 | 1.853–55.249 | 0.008 |
| RPR before to after treatment | |||
| Low to low | 1 | ||
| Low to high | 132.6 | 3.689–4,767.341 | 0.007 |
HR, hazard ratio; CI, confidence interval; LV, lymphovascular; HER2, human epidermal growth factor receptor 2; RDW, red blood cell distribution width; COV, cut-off value; RPR, red blood cell distribution width to platelet count ratio.
Discussion
In our study, we suggested that elevated post-treatment RDW and RPR were associated with poor prognosis in patients with breast cancer, and these may be used as prognostic factors in cases of both high RDW and high RPR. To the best of our knowledge, this is the first study to evaluate the effects of RDW with RPR before and after treatment. In particular, our results demonstrated that elevate RDW and RPR values before and after treatments were independent factors for poor survival in breast cancer patients.
In cancer microenvironment, inflammation promotes tumor growth, invasion, angiogenesis, and eventually metastasis (14,15). During inflammation, red blood cell maturation disturbs the red blood cell membrane, leading to increased RDW (16). The mechanism underlying the relationship between RDW and survival or disease activity is not clear. However, high RDW thought to be caused by chronic inflammation, age-related diseases, and oxidative stress, which lead to changes in erythropoiesis (4,17). Because a malignant tumor may extend the inflammatory response in the process of its progression and increase circulation levels, RDW may be a potential biomarker of cancer growth and metastatic activity (18). In addition, RDW is an easily available and inexpensive parameter in blood samples and reflects the size heterogeneity of circuiting erythrocytes. Therefore, preoperative RDW was suggested as an independent predictor of breast cancer in a recent retrospective study (18).
Platelet count is a parameter that is measured in the complete blood count and is affected by systemic inflammation. A study showed that elevated platelet levels can be seen in many cancers, and that platelet count is inversely correlated with survival (19). Platelets are rich in growth factors such as platelet-derived growth factor-receptor (PDGF), transforming growth factor-beta. The PDGF-receptor is involved in cancer invasion and metastasis (20). In breast cancer, PDGF beta receptor expression is correlated with unfavorable pathological characteristics and survival (20).
In several studies, elevated RPR levels have been associated with poor prognosis in chronic hepatitis, pancreatitis, and acute myocardial infarction (12,21,22). Although the reason for this is uncertain, the imbalance between RDW and platelet count could be a significant prognostic factor for the poor prognosis of breast cancer patients with elevated RPR (13). Most of the aforementioned studies used preoperative RDW and PRP values, and significant values were obtained. However, in our study, there was no significant difference before treatment with RDW and RPR. Rather, only the after-treatment RDW and RPR values were significantly associated with survival. Therefore, we analyzed two points. One is that dividing different subgroups that one is high RDW/high RPR group which satisfies both high RDW and high RPR group and another is low RDW/low RPR group which satisfies both low RDW and low RPR group. Another point is that the RDW and RPR changed after initial treatments between the lower and higher groups. In this analysis, we revealed that when a lower value before treatment became higher after treatment, survival was very poor.
There are limitations in our study that were conducted in a single center, and it was performed retrospectively with a limited number of patients. In particular, in OS, it can be very difficult to conclude as the final death is only 14. Although the absolute values of RDW and RPR are currently difficult to determine, the overall elevated values are meaningfully available for each hospital. In addition, it is necessary to investigate patients with breast cancer who undergo neoadjuvant chemotherapy.
Recently, various blood cell markers have been reported to be prognostic factors. So, further study on details including mechanism or intervention should be carried out.
To our knowledge, the present study is the first to reveal a relationship between RDW, RPR levels in before and after treatment, and survival. The results of our study confirmed that observing the RDW, RPR levels in before-after treatment, and the process of continuous change can be important indicators for predicting the prognosis of breast cancer patients.
Conclusions
Elevated RDW and RPR levels after surgery and adjuvant therapy are associated with poor DFS and OS in patients with breast cancer. Particularly, when both the high RDW and high RPR groups were satisfied, the prognostic value was significantly associated with poor survival. Furthermore, when the RDW and RPR groups had lower levels before treatment and then increased to a higher level after treatment, the prognostic value was more significantly associated with poor survival. As RDW and RPR are simple and cost-effective, they are useful biomarkers for improved risk assessment in breast cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-410/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-410/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-410/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-410/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital (No. GNUCH 2021-11-013) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benson JR, Jatoi I. The global breast cancer burden. Future Oncol 2012;8:697-702. [Crossref] [PubMed]
- Kuniyoshi RK, Gehrke Fde S, Alves BC, et al. Gene profiling and circulating tumor cells as biomarker to prognostic of patients with locoregional breast cancer. Tumour Biol 2015;36:8075-83. [Crossref] [PubMed]
- Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med 2016;4:399. [Crossref] [PubMed]
- Förhécz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009;158:659-66. [Crossref] [PubMed]
- Cui MM, Li N, Liu X, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep 2017;7:3456. [Crossref] [PubMed]
- Zhang X, Cui MM, Fu S, et al. Platelet distribution width correlates with prognosis of gastric cancer. Oncotarget 2017;8:20213-9. [Crossref] [PubMed]
- Suzuki K, Aiura K, Kitagou M, et al. Platelets counts closely correlate with the disease-free survival interval of pancreatic cancer patients. Hepatogastroenterology 2004;51:847-53.
- Long Y, Wang T, Gao Q, et al. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: a meta-analysis. Oncotarget 2016;7:81849-61. [Crossref] [PubMed]
- Pietrzyk L, Plewa Z, Denisow-Pietrzyk M, et al. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac J Cancer Prev 2016;17:4433-7.
- Ekici H, Malatyalioglu E, Kokcu A, et al. Do Leukocyte and Platelet Counts Have Benefit for \Preoperative Evaluation of Endometrial Cancer? Asian Pac J Cancer Prev 2015;16:5305-10. [Crossref] [PubMed]
- Qiu J, Yu Y, Fu Y, et al. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res 2012;38:651-7. [Crossref] [PubMed]
- Taefi A, Huang CC, Kolli K, et al. Red cell distribution width to platelet ratio, a useful indicator of liver fibrosis in chronic hepatitis patients. Hepatol Int 2015;9:454-60. [Crossref] [PubMed]
- Takeuchi H, Abe M, Takumi Y, et al. Elevated red cell distribution width to platelet count ratio predicts poor prognosis in patients with breast cancer. Sci Rep 2019;9:3033. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Allen MD, Jones LJ. The role of inflammation in progression of breast cancer: Friend or foe? Int J Oncol 2015;47:797-805. (Review). [Crossref] [PubMed]
- Demirkol S, Balta S, Cakar M, et al. Red cell distribution width: a novel inflammatory marker in clinical practice. Cardiol J 2013;20:209. [Crossref] [PubMed]
- Sousa R, Gonçalves C, Guerra IC, et al. Increased red cell distribution width in Fanconi anemia: a novel marker of stress erythropoiesis. Orphanet J Rare Dis 2016;11:102. [Crossref] [PubMed]
- Yao D, Wang Z, Cai H, et al. Relationship between red cell distribution width and prognosis in patients with breast cancer after operation: a retrospective cohort study. Biosci Rep 2019;39:BSR20190740. [Crossref] [PubMed]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237-49. [Crossref] [PubMed]
- Paulsson J, Sjöblom T, Micke P, et al. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am J Pathol 2009;175:334-41. [Crossref] [PubMed]
- Cetinkaya E, Senol K, Saylam B, et al. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol 2014;20:14450-4. [Crossref] [PubMed]
- Pusuroglu H, Cakmak HA, Akgul O, et al. The prognostic value of admission red cell distribution width-to-platelet ratio in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Rev Port Cardiol 2015;34:597-606. [Crossref] [PubMed]


