
Establishment of a Bama miniature pig burn model with different burn depths
Introduction
The incidence of burn injury, for which the elderly and children comprise high-risk groups, is increasing year by year (1). In hospitalized children with accidental injuries, burn injury ranks 2nd among the causes of injury (2). The annual incidence rate of burn injury in China ranges from about 1.5–2%; that is, 20 million people suffer from different degrees of burns, of whom about 5% require hospitalization (3). In addition to having a considerable incidence rate, severe burns also cause disfigurement or even accidental death. Burns can be divided into the following 3 degrees and 4 levels according to damage severity: first-, second- and third-degree burns, of which second-degree burns can be further divided into superficial second-degree burns and deep second-degree burn. First-degree, superficial second-degree, and deep second-degree burns generally take about 5–7 days, 2–3 weeks, and 3–4 weeks to heal, respectively. However, some of the more serious deep second-degree burn and third-degree burns require a longer time to repair, and some cannot even heal naturally, and require surgical skin grafting (4).
There have been significant advances in burn treatments; however, a new, effective treatment mode and drug still need to be developed. A large and ideal mammalian model with different degrees of burns needs to be established to observe skin repair, study the burn mechanism, and evaluate the potential of novel therapeutics and drugs. An animal model should be selected that is similar to the human structure, function, metabolism style, and disease characteristics. To date, mice (5), rats (6), rabbits (7), pigs (8), and dogs (9) have been commonly used to establish burn models; however, each has various problems.
The skin structure of the Bama miniature pig is very similar to that of the human in morphology, physiology, and pharmacology, including in relation to hair density, epidermal thickness, and humoral and metabolic changes in burned skin (10,11). Importantly, the burned skin of both pigs and humans repair through epithelization, so it is an ideal animal model for researching burn mechanism and evaluating novel therapeutics, and the data might provide theoretical foundation for further clinical transformation application. But the model also has a number of disadvantages, including high costs, and difficulties associated with the large size of the animals, and the complexity of the operation.
Depth-burn models with large animals, such as pigs and dogs, are prepared with combustible liquids, such as absolute ethanol, combustible solids, such as jellied gasoline, thermal burns, such as hot-water burns, steam-induced burns, copper column boiled with hot-water burns, electrothermal burns, flame burns, radiation burns, chemical burns, and inhalation burns (12,13). However, these methods have different limitations, including unsteady temperatures, uncontrollable pressure on the contact surface, and inconsistent burn depth. In this study, a Bama miniature pig burn model with different degrees of burns was prepared using a new innovative electronic burn instrument (ZL201920043476.1). The new electronic burn instrument is characterized by accurate temperature control, uniform depth, and a controllable range of burn injury, and a more stable and reliable burn animal model was established using this burn instrument compared with other method. Our findings provide a basis for further research on the burn mechanism and evaluations of therapeutic drugs. We present the following article in accordance with the ARRIVE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-476/rc).
Methods
Animal care
All the experiments adhered to procedures consistent with the International Guiding Principles for Biomedical Research Involving Animals issued by the Council for the International Organizations of Medical Sciences (CIOMS) and were approved by the Institutional Animal Care and Use Committee at The Fourth Medical Center of PLA General Hospital (the ethics approval ID: 2021KY033-KS001). A protocol was prepared before the study without registration. A total of 6 ordinary male Bama miniature pigs were purchased from the Beijing Strong Century Minipigs Breeding Base. The minipigs were housed at the Laboratory Animal Center of The Fourth Medical Center of PLA General Hospital (Beijing, China), and kept on a 12:12-h light–dark cycle, at room temperature maintained between 20 and 22 with a humidity range of 50–60%, for 1 week before the experiment for adaptation.
Establishment of a Bama miniature pig burn model with different degrees
When the 6 male Bama miniature pigs reached a weight of 23–28 kg and had a mean length of 71–75 cm, they were used to make burn models. Before the experiment, the hair of each pig was shaved and depilated on the back. Each pig was then anesthetized with an intramuscular injection of 1 mg of Ketamine and Sumianxin II. The anesthesia was maintained with 3% Pentobarbital sodium (0.2 mL/kg) as needed. To obtain consistent and controllable burn wounds, a new electronic burn instrument (ZL201920043476.1) was invented by our team (see Figure 1). In this study, 8 round burn wounds with a diameter of 5 cm and different burn degrees were prepared separately on both sides of each pig’s back by applying a continuous pressure of 1 kg perpendicular to the spine using the electronic burn instrument at 100 °C. The different burn degrees were determined by the contact time of the electronic burn instrument, which were as follows: 0 s (control), 10 s, 15 s, 20 s, 25 s, 30 s, 35 s, 40 s, and 45 s. The physiological state and burn wounds of the Bama miniature pigs were then closely observed.
Specimen collection and preparation of formalin-fixed paraffin-embedded (FFPE) tissue slides
The skin burn tissue on both sides of each pig’s back was cut at 24 h after the burn to the muscular layer. The cut site was in the middle of the burn wound. The skin samples were divided into small pieces, rinsed with phosphate buffered solution and fixed in 4% paraformaldehyde for 7 d at room temperature. Subsequently, the skin burn tissue was immersed in gradient ethanol, ethanol-xylene mixture, pure xylene, and pre-melted paraffin wax 1 by 1 to embed the skin burn tissue into paraffin. The paraffin specimens were sectioned in a plane perpendicular to the incision, while the section thickness was 5 µm.
Hematoxylin and eosin (H&E) staining
The sections of burn tissue for 0 s (control), 10 s, 15 s, 20 s, 25 s, 30 s, 35 s, 40 s, and 45 s were successively immersed 1 by 1 in dimethylbenzene, 2 times for 10 min each time. The deparaffinized sections were immersed in gradient ethanol and then rehydrated by flowing distilled water, 3 times for 1 min each time, stained with hematoxylin dye solution for 5–10 min, and stained with eosin solution for 5 min, dehydrated in gradient ethanol, and hyalinized in pure xylene 3 times for 2 min each time, and finally sealed by the appropriate amount of neutral gum. After drying, the skin morphology was observed under an optical microscope (Olympus, USA).
Masson staining
The pre-treatment of the sections of burn tissue was consistent with that of H&E staining. The pre-treated sections were immersed in potassium dichromate overnight at room temperature and then stained in hematoxylin solution for 3–5 minutes. After being washed with flowing distilled water for 1–3 min, the blue was reversed by immersing them in ethanol-hydrochloric acid solution. Subsequently, the sections were transferred into a dye solution composed of ponceau and acid fuchsin for 10–15 min, phosphomolybdic acid aqueous solution for 3–5 min, aniline blue staining solution for 6 min, glacial acetic acid (1% v/v) for 2 min, and 95% ethanol and xylene for 5 s. The sections were dried in a ventilated place, and then covered with a cover slip with neutral balsam. After drying, the sections were observed under an optical microscope (Olympus, USA); blue indicated normal collagen fiber structure, and red indicated collagen fiber degeneration and necrosis.
Results
A new electronic burn instrument
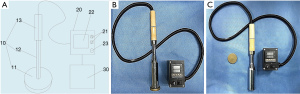
Previous studies have shown that the methods for making burn models have the disadvantages of an uncontrollable and unstable temperature, contact pressure, and burn area, which result in inconsistent burn degrees and poor experimental repeatability. Thus, a portable electronic burn instrument was invented according to the specific experimental requirements, for which a patent has been applied for in China (No. ZL 201920043476.1). The mechanical structure of this instrument is shown in Figure 1A,1B. The copper plate of the electronic burn instrument that comes in direct contact with the pig skin is removable, and it uses double electron tubes for heating, and thus has a rapid temperature rise of <1 min. The temperature of the burn components is constant, and the change is very low after contact with the pig skin and can be controlled within ±0.1 °C. The temperature control element is removable and has a variety of burn components, of which the shape can be round or square, and the size of burned component ranges from 1–10 cm (see Figure 1C). The contact pressure of the electronic burn instrument on the pig skin can also be adjusted to establish pig models with different burn degrees.
Thus, the new electronic burn instrument solved the problems related to an accurate temperature, pressure control and a consistent burn degree, and thus ensured the good repeatability and consistency of the experiment.
Preparation of the Bama miniature pig burn model
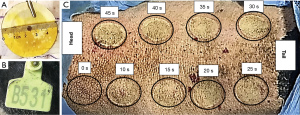
In total, 6 Bama miniature pigs were used to establish the structure burn model using the new electronic burn instrument. The injury conditions were as follows: 100 °C, continuous pressure of 1 kg perpendicular to the spine, a wound surface diameter of 5 cm (see Figure 2A), contact for 0 s (control), 10 s, 15 s, 20 s, 25 s, 30 s, 35 s, 40 s, and 45 s, respectively. Finally, we made 8 wounds with different burn degrees on both sides of the back of each pig with its own earmark (see Figure 2B). As Figure 2C shows, the wound sizes prepared at the different contact times were nearly the same and the wound edges were neat without ulceration and infection. No obvious blisters were observed to form in any of the burn wound tissues due to the thick cuticle of the pig skin, but the burn marks of pig skin gradually deepened with the extension of the burn time.
H&E staining
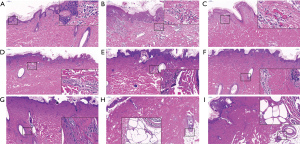
To further determine the depth of the burn, we observed the histopathological changes of the burned skin tissues by H&E staining detection. As Figure 3A shows, for normal skin (i.e., skin with a burn time of 0 s), the skin structure maintained its integrity and the collagen fibers were arranged densely and orderly. As Figure 3B,3C show, for the first-degree burns (i.e., skin treated at 100 °C for 10 and 15 s), the blood vessels in the superficial dermis were dilated and congested, and necrosis occurred above the basal layer of the epidermis. As Figure 3D,3E show, for the superficial partial-thickness burns (i.e., skin treated at 100 °C for 20 and 25 s), the whole epidermal layer was necrotic, and the dermal papilla layer was also severely damaged. For the deep partial-thickness burns, (i.e., skin treated at 100 °C for 30 and 35 s), the epidermis and dermis were necrotic with leukocyte infiltration zones, but the skin; appendages in the deep dermis remained alive (see Figure 3F,3G). For the third-degree burns, (i.e., skin treated at 100 °C for 40 and 45 s), the skin layer and adipose tissue were all necrotic, thick blood vessels could be observed in the skin adipose tissue, and were filled with disintegrating and agglutinating red blood cells (see Figure 3H,3I). Thus, treatment at 100 °C for 30 s or more resulted in deeper skin burn injuries.
Masson staining
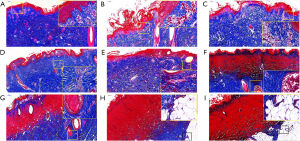
Masson staining was performed to observe the collagen fiber structure changes of the burned skin. Consistent with the H&E staining results, the Masson staining results showed that the epidermis and dermis structure of the normal skin was also complete, and the skin injury became more and more serious with the extension of the burn time. The collagen fibers were arranged neatly in normal skin (i.e., skin treated at 100 °C for 0 s; see Figure 4A), and there was almost no damage to the collagen fibers in the first-degree burns. Consistent with the H&E results, the Masson results also clearly showed that the blood vessels in the superficial dermis were dilated and congested, and necrosis occurred above the basal layer of epidermis in the first-degree burns (i.e., skin treated at 100 °C for 10 and 15 s; see Figure 4B,4C). In the superficial partial-thickness burns (i.e., skin treated at 100 °C for 20 and 25 s), the collagen fibers were slightly disordered and injured (see Figure 4D,4E). In the deep partial-thickness burns (i.e., skin treated at 100 °C for 30 and 35 s), the structure of collagen fibers was partially degenerated and necrotized (see Figure 4F,4G). In the third-degree burns (i.e., skin treated at 100 °C for 40 and 45 s), the collagen fibers were severely degenerated, or even completely necrotized (see Figure 4H,4I). The Masson staining results indicated that the collagen fibers were severely damaged with treatment at 100 °C for 30 s or more.
Discussion
Burns are a global health problem and cause serious physical and psychological harm to patients, especially children and the elderly. At present, about 265,000 people worldwide have died of burns (14). It has been reported that the incidence of burns was 0.19% in the United States, 2.43% in Southeast Asia, and 1.87% in the Mediterranean (15-18). In China, >20 million patients suffer from different degrees of burns (19). With the continuous development and improvement of burn treatments, most burn patients can enter the rehabilitation period smoothly. However, the dysfunction and disfigurements caused by scar contracture still occur frequently in the process of rehabilitation (20). Thus, the development of effective burn treatment methods and drugs is a research hotspot. A burn animal model provides the necessary basis for burn research. The selection of an appropriate burn animal model largely depends on practical problems, such as the experimental purpose, experimental condition, and experimental cost.
Bama miniature pigs are ideal to establish experimental animal modes to study skin burns, as they are similar to humans in terms of their body size, their physiological condition, and disease development. Most of the skin of Bama miniature pig is covered with white hair, which is convenient for experimental observation and operation (11). According to Liu, the skin morphological characteristics of the Bama miniature pig are similar to those of the human, including in relation to the thickness of the skin layer, the development of the surface vascular system, the structure of the dermal epidermal interface, and the extracellular matrix (10). However, the skin of Bama miniature pigs lacks many antigenic determinants corresponding to those of the skin of humans, and there is no cross reaction with human antiserum, which is helpful to further study the immunological characteristics of human skin (10). Additionally, the drug metabolism process of Bama miniature pig skin is similar to that of human skin, so the applicability, dose safety, and toxicological characteristics of burn drugs can be evaluated well (21). Thus, Bama miniature pig skin has unique value in skin research.
In the establishment of an animal burn model, it is difficult to determine the precise area and depth of injury due to differences in each animal body shape and skin structure/thickness. Thus, it is necessary to individualize the injury and clarify the burn depth in combination with wound conditions and pathological examinations. Previously, there was no standardized, high-precision and controllable effective method for preparing a Bama miniature pig burn model. Common methods for preparing burn models of pigs, dogs, and other large animals include thermal burns (hot-water burns, steam burns, and hot-metal burns), electric-light source burns, flame burns (burns with combustible liquids, such as absolute ethanol, and combustible solids such as solidified gasoline powder), electric burns, radiation burns, chemical burns, and inhalation burn. However, these methods have various limitations, such as unstable temperatures, uncontrollable contact pressures, and inconsistent burn depths (12,13). For example, when a copper column heated by boiling water comes in contact with animal skin, the copper column cools, and thus the temperature drops. Similarly, a combustible liquid may extend to an unexpected, undesignated skin area, making it difficult to control the burn area. Additionally, the previous methods for preparing Bama miniature pig burn models were performed mechanically according to the preparation conditions of other animal models, which have not been explored or improved.
To overcome these defects, a new electronic burn instrument was invented. The heated copper plate of the new electronic burn instrument comes in direct contact with the pig skin, so the area and location of burns are strictly controlled. The copper plate is removable, the size ranges from 1–10 cm, and can be round or square in shape; thus, the size of the burn area can be flexibly adjusted, preventing an insufficient burn depth caused by too small a burn area, and a systemic reaction caused by a too large burn area. The copper plate of the new electronic burn instrument that comes in direct contact with the pig skin is heated by double electron tubes. The temperature of the whole copper plate is uniform, constant, and changes little after coming in contact with the pig skin, and can be controlled within ±0.1 °C. Additionally, the contact pressure of the copper plate on the pig skin can be adjusted according to the experimental purpose and physiological state of the Bama miniature pigs. The precise copper plate temperature and contact pressure on the pig skin ensure the consistency and controllability of the burn depth.
The new electronic burn instrument is suitable for preparing burn models of Bama miniature pigs, and other large animals. Additionally, the preparation conditions of an animal burn model, including the copper plate temperature, contact pressure on the skin, and burn area, need to be explored to prepare the different burn depths to meet the experimental designs of different studies. Further, the histopathological changes after injury at different times should be described to stabilize the animal model for further mechanism research and new treatment evaluations (22). The deep partial-thickness and third-degree burns belong to deep burns in clinic, and burned wound healing is relatively slow, leaves severe scars, and may require skin grafting operation. Burn severity generally directly correlates to the total body surface area (TBSA) and burned deep of the injured tissue. Above 30% TBSA of deep burns in adult and 10% TBSA of deep burns in children, they are considered as severe burn and can induce multiple organ injury and excessive systemic inflammation. Severe burn is a complex and severe traumatic injury with high mortality in the acute phase, and prompt and appropriate treatment of severe burns is necessary, especially in the phase. In this study, the results showed that the preparation condition of 100 °C, a continuous pressure of 1 kg perpendicular to the spine, and a contact time with the pig skin of 30 s or more was optimum for establishing deeper burns in the Bama miniature pigs. Notably, pre-experiments were necessary to adjust the burn conditions according to the physiological state and age of the Bama miniature pigs and the experimental purpose.
In conclusion, this study provided an accurate and controllable stabilization method for the preparation of Bama miniature pig models with different burn depths using a newly invented electronic burn instrument. Further, these burn models provide a basis for further research on the burn mechanism and evaluations of therapeutic drugs.
Acknowledgments
Funding: This work was supported by the Major Project of Military Logistical Support Department (Nos. ALB19J001, AWS15J003), the Natural Science Foundation of Beijing (No. 7222179), and the National Natural Science Foundation of China (No. 81772067), Health and Technology of Inner Mongolia (No. 202202146).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-476/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-476/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-476/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the experiments adhered to procedures consistent with the International Guiding Principles for Biomedical Research Involving Animals issued by the Council for the International Organizations of Medical Sciences (CIOMS) and were approved by the Institutional Animal Care and Use Committee at The Fourth Medical Center of PLA General Hospital (the ethics approval ID: 2021KY033-KS001).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riedlinger DI, Jennings PA, Edgar DW, et al. Scald burns in children aged 14 and younger in Australia and New Zealand—an analysis based on the Burn Registry of Australia and New Zealand (BRANZ). Burns 2015;41:462-8. [Crossref] [PubMed]
- Jin Y, Duan L, Wang Y, et al. 844 Trend and current characteristics of burn from chinese national injury surveillance system from 2006 to 2013. Inj Prev 2016;22:4. [Crossref]
- Sheng Z, Guo Z. Editors. Critical burn treatment and rehabilitation. First edition. Beijing: Science Press; 2000:136-7.
- Itakussu EY, Morita AA, Kakitsuka EE, et al. Instruments to assess function or functionality in adults after a burn injury: A systematic review. Burns 2021;47:999-1011. [Crossref] [PubMed]
- Zhang T, Zhang R, Xu B, et al. Spinal endomorphins attenuate burn-injury pain in male mice by inhibiting p38 MAPK signaling pathway through the mu-opioid receptor. Eur J Pharmacol 2021;903:174139. [Crossref] [PubMed]
- Shen C. 650 Exploring the Mechanism of Wound Healing in Rats of Different Ages Using a Burn Model. Journal of Burn Care & Research 2020;S171. [Crossref]
- Djerrou Z, Maameri Z, Hamdi-Pacha Y, et al. Effect of virgin fatty oil of Pistacia lentiscus on experimental burn wound's healing in rabbits. Afr J Tradit Complement Altern Med 2010;7:258-63. [Crossref] [PubMed]
- Sun Y, Cao Y, Zhao R, et al. The Role of Autologous PRP on Deep Partial-Thickness Burn Wound Healing in Bama Pigs. J Burn Care Res 2020;41:657-62. [Crossref] [PubMed]
- Atiba A, Ghazy A. The Effects of Topical Dimethyle Sulfoxide on Second-Degree Burn Wound Healing in Dogs. Alex J Vet Sci 2015;45:6-12. [Crossref]
- Liu Y, Chen JY, Shang HT, et al. Light microscopic, electron microscopic, and immunohistochemical comparison of Bama minipig (Sus scrofa domestica) and human skin. Comp Med 2010;60:142-8. [PubMed]
- Chen JY, Hu J, Wei H. Comparative Study on Skin between BAMA Miniature Pig and Human. Chinese Journal of Comparative Medicine 2006;16:288-91.
- Borman H, Bilezikci B, Maral T. The use of superficial and deep partial-thickness burned tissue for flap fabrication. Burns 2007;1:S53-4. [Crossref]
- Shukla SK, Sharma AK, Shaw P, et al. Creation of rapid and reproducible burn in animal model with a newly developed burn device. Burns 2020;46:1142-9. [Crossref] [PubMed]
- Forbinake NA, Ohandza CS, Fai KN, et al. Mortality analysis of burns in a developing country: a CAMEROONIAN experience. BMC Public Health 2020;20:1269. [Crossref] [PubMed]
- Sorenson TJ, Mohr WJ, Mahajan AY. Pediatric Hand Burns Requiring Emergency Care in the United States. J Burn Care Res 2021; Epub ahead of print. [Crossref] [PubMed]
- Van Niekerk A, Rode H, Laflamme L. Incidence and patterns of childhood burn injuries in the Western Cape, South Africa. Burns 2004;30:341-7. [Crossref] [PubMed]
- Othman N, Kendrick D. Epidemiology of burn injuries in the East Mediterranean Region: a systematic review. BMC Public Health 2010;10:83. [Crossref] [PubMed]
- Van Yperen DT, Van Lieshout EMM, Verhofstad MHJ, et al. Epidemiology of burn patients admitted in the Netherlands: a nationwide registry study investigating incidence rates and hospital admission from 2014 to 2018. Eur J Trauma Emerg Surg 2022;48:2029-38. [Crossref] [PubMed]
- Zhu L, Zhang H, Shi F, et al. Epidemiology and outcome analysis of scalds in children caused by "guo lian kang": an 11-year review in a burn center in China. Burns 2015;41:289-96. [Crossref] [PubMed]
- Hawkins H, Jay J, Finnerty CC. Pathophysiology of the Burn Scar. Total Burn Care (Fifth Edition) 2018:466-75.e3.
- Liu Y, Zeng BH, Shang HT, et al. Bama miniature pigs (Sus scrofa domestica) as a model for drug evaluation for humans: comparison of in vitro metabolism and in vivo pharmacokinetics of lovastatin. Comp Med 2008;58:580-7. [PubMed]
- Davenport L, Dobson G, Letson H. A new model for standardising and treating thermal injury in the rat. MethodsX 2019;6:2021-7. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


