
A clinical diagnostic test on the detection of sentinel lymph node metastasis in breast neoplasms using a 1-step RT-PCR
Introduction
Breast cancer is a malignant tumor with a high incidence rate among women in my country (1). In recent years, the incidence of breast tumors has increased significantly, and the micro-metastases can occur at an early stage. Metastasis is an important biological characteristic of malignant tumors, and it is also the root cause of clinical intractability (2). Thus, the timely diagnosis of metastases and their proper treatment is crucial. Axillary lymph node status is the most valuable indicator of clinical diagnosis and treatment, and has an important reference value. The concept of sentinel lymph nodes (SLNs) was first proposed by Cabanas in 1977 (3). SLNs refer to lymph nodes that first receive lymphatic drainage in the tumor region. As more and more breast cancer gets screen-detected at an early stage, sentinel lymph node biopsy (SLNB) has emerged as a most predominant tool to probe axillary lymph node status for patients who have clinically negative lymph nodes (2). The status of SLNs accurately reflects whether lymph node metastasis has occurred in axillary lymph nodes (4). Thus, the molecular detection of SLNs in breast cancer is of great significance, and can lead to the early diagnosis of the disease.
At present, the routine pathological diagnosis of SLN includes rapid intraoperative frozen section analyses and touch imprint cytology, which are widely used either alone or in combination. However, these detection methods have limited abilities to detect tissue and cannot detect intact lymph node status. The biggest disadvantage of these routine tests relates to their lack of sensitivity to micro-metastatic SLNs. The rapid intraoperative diagnosis of SLN is crucial, and current intraoperative frozen section analyses take a long time to complete. Thus, breast lymph node (BLN) molecular detection, which is an objective, standardized and repeatable detection technology, is receiving more and more attention.
If SLNs can be evaluated during breast cancer surgery, secondary surgery could be avoided. Commonly used methods for the evaluation of SLNs include visual inspections, cytology, frozen section analyses, and combinations of these methods; however, all these methods have low sensitivity, can damage the specimens, and are time consuming. At present, pathological methods for the evaluation of SLNs metastasis require considerable sensitivity and specificity and must also be rigorously comprehensive, and able to make a total assessment of the resected SLNs. However, SLNs diagnoses of frozen tissue sections require time, labor, and experienced pathologists and medical technicians which are critical in ensuring accurate diagnoses. However, such SLN diagnosis methods also have some disadvantages, including that they are time consuming, have limited detection abilities, and lack objectivity and reproducibility. If, for example, a standard, single level HE section identifies 25% of SLNs as positive, this will increase to 40% if further levels and/or immunohistochemistry are performed and 2–20% of cases are upstaged if such ancillary techniques are used (5). Routine hematoxylin and eosin (HE) staining of small metastases (with a diameter <2 mm) can lead to missed diagnoses in SLN biopsy results due to false-negative results.
In recent years, molecular detection technology has attracted attention. Reverse transcription-polymerase chain reaction (RT-PCR) has the advantages of high specificity, high accuracy, high sensitivity. RT-PCR is highly sensitive and specific method for the detection of metastasis. It can detect metastasis in lymph nodes with a diameter >0.2 mm, and can detect metastasis in the blood system, lymph nodes, and bone marrow. Thus, as an objective, standardized and repeatable detection technology, BLN molecular detection is receiving more and more attention. A meta-study systematically evaluated the clinical efficacy and cost-effectiveness of one-step nucleic acid amplification (OSNA) and Metasin in the intraoperative analysis of SLN metastasis in breast cancer patients. And proposed that the OSNA test only analyzes cytokeratin 19 (CK19) gene expression, while the Metasin analysis analyzes CK19 and mammaglobin gene expression (6). In this study, we focused more on intraoperative analysis of the plausibility of the expression of two markers in sentinel lymph nodes as biomarkers for the presence of metastases.
This study used RT-PCR to detect mammaglobin and CK19 messenger ribonucleic acid (mRNA) in breast cancer SLNs. The purpose of this study was to explore the clinical application of this method in SLNs assessments, and its use in guiding clinical treatment plans. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-485/rc).
Methods
Patients
This study prospectively collected 256 sentinel lymph nodes from 150 patients diagnosed with breast cancer between August and November 2017 in the Department of Pathology at The Fourth Hospital of Hebei Medical University. One hundred fifty female patients were enrolled in the study. This study was approved by Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2018HEC153), and complied with the Declaration of Helsinki (as revised in 2013). All patients signed the informed consent form. The median age of onset was 50 years old (range, 26–70 years old). Of the 150 patients, 25 had intraductal carcinoma with micro-infiltration, 109 had invasive ductal carcinoma, 5 had invasive lobular carcinoma, 3 had breast infiltrating micropapillary carcinoma, 2 had breast mucinous adenocarcinomas, 2 had spindle cell metaplastic carcinoma of the breast, 1 had breast cancer with myeloid features, 1 had mammary glands with apocrine gland differentiation, 1 had mammary glands with neuroendocrine features, and 1 had Paget’s disease. The 256 SLNs were removed from the 150 patients during surgery, and the median number of SLNs per person was 2. There were 41 positive lymph nodes with a positive rate of 16.02%. The SLNs were positive in 27 of the 150 patients. Among the 256 SLNs, there were 27 macro-metastatic lymph nodes, 6 micro-metastatic lymph nodes, 8 isolated lymph node cells, and 215 negative lymph nodes according to the immunohistochemistry (IHC) results. Immunohistochemical sections were stained with streptavidin-peroxidase (SP) method. In this study, IHC results were used as the gold standard for judging sentinel lymph node status.
Study methods
Processing and evaluation of specimens
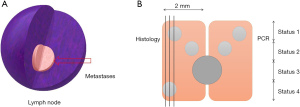
The SLNs were clinically examined perpendicular to the long axis of the lymph nodes, and cut at 2 mm intervals. The odd-numbered blocks were frozen and subjected to subsequent gene molecular detection, and the even blocks were permanently serially sliced. Each odd-numbered lymph node was placed in a 1.5-mL de-nuclease-water centrifuge tube to which liquid nitrogen was quickly added. Each tube was then immediately stored at −80 °C for a long period. Throughout the entire process, any cross-contamination of the epithelial cells was strictly avoided.
Division principle and calculation method
The number of tumor cells in a metastatic lesion was based on the 8th edition of the American Joint Committee on Cancer (AJCC) tumor staging guidelines, and the diameter of a single tumor cell was 0.01 mm. Isolated tumor cells (ITCs) were defined as lymph node metastasis <0.2 mm, and no more than 200 tumor cells in a single lymph node. Micro-metastasis was defined as lymph node metastasis >0.2 mm, and <2 mm. Macro-metastasis was defined as lymph node metastasis >2 mm. Therefore, the micro-diafiltration volume was calculated to have a diameter of about 0.2 mm, which should be 203=8,000 cells, and the micro-transition/macro transfer limit was: (200)3=8,000,000 cells.
PCR method and threshold determination
Threshold determination
The number of tumor cells in the metastases was set and the threshold of metastases in the RT-PCR under different conditions was determined according to the 8th edition of the AJCC tumor staging guidelines. In relation to the different SLN metastases, different numbers of breast cancer T47D cell lines were infiltrated by cell cultures, and the metastases of SLNs were determined according to differences in CT values.
RNA extraction
Total RNA was extracted from fresh lymphoid tissue using the Qiagen No. 74104 RNeasy Mini Kit. The ratio of OD260/OD280 was measured by a spectrophotometer to verify the purity of the RNA, and the ratio was >1.8 for RT-PCR detection.
Primer selection
The sequence was synthesized by YingjieJieji (Shanghai) Trading Co. The primer sequences were as follows:
- CK19 upstream primer: 5'-CCATTGAGAACTCCAGGATTG-3';
- CK19 downstream primer: 5'-CTTGGTTCGGAAGTCATCTG-3;
- Mammaglobin upstream primer: 5'-CTTCAAGAGTTCATAGACGACA-3';
- Mammaglobin downstream primer: 5'-GAGTTTCATCCGTTTGGTTAAG-3';
- Internal reference upstream primer: 5'-ATTCCACCCATGGCAAATTC-3';
- Downstream primer: 5'-GATGGGATTTCCATTGATGACA-3'.
1-step RT-PCR amplification
Subsequent experiments were performed using the RR064A 1-step PCR kit from TaKaRa in Japan. Total RNA was thawed on ice and tested for RNA concentration and diluted to 20 ng/µL. The RT-PCR amplification conditions were as follows: 10 µL per well system, 5 µL of RT-PCR reaction buffer, 0.2 µL of deoxy-ribonucleoside triphosphate (dNTP), 0.4 µL of primer probe, 0.2 µL of thermus aquaticus (Taq) enzyme, 3.2 µL of de-nuclease water, and 1 µL of RNA. The a1mplification procedure was as follows: 42 °C for 5 min; 95 °C for 10 s; 95 °C for 2 s; 60 °C for 3 s (for 39 cycles). Mix the above materials well and transfer to 96-well plates, with 3 replicate wells per gene.
Statistical analysis
The analysis and processing were performed using statistical software SPSS 22.00, and in this study, IHC results were used as the gold standard for judging sentinel lymph node status. The overall consistency, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the two assays were also determined. The statistical analyses were performed using chi-square tests. The P<0.05 was considered statistically significant.
Results
Culture breast cancer T47D cell line
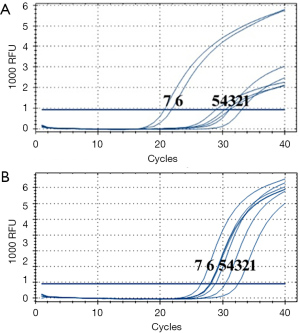
Macroscopic metastases, micro-metastases, ITCs, and negative lymph node-built cell models were implanted into breast cancer T47D cells (Figure 1). The RT-PCR CT values of the 7 groups showed an increasing trend. For the macro-metastases, micro-metastases, ITCs, and negative lymph node models, the CT values of RT-PCR increased. The CK19 CT values for each of the 7 groups were 33.06±0.045, 31.12±0.036, 31.09±0.076, 30.34±0.083, 28.91±0.355, 21.81±0.067, 20.65±0.523, respectively. The mammaglobin CT values for each of the 7 groups were 32.46±0.346, 30.39±0.089, 28.83±0.074, 28.12±0.062, 28.03±0.065, 27.98±0.523, and 26.53±0.531, respectively (Figure 2). The mammaglobin ΔCT value of the macro-metastasis positive sample in the 7 groups of samples was 2.98±0.67, and the CK19 ΔCT value was 2.23±1.12. The micro-metastasis positive sample mammaglobin ΔCT value was 3.56±0.43 and the CK19 ΔCT value was 2.79±0.89. The ITC positive samples had a mammaglobin ΔCT value of 3.55±0.78, and the CK19 ΔCT value was 3.12±1.23. The negative sample mammaglobin ΔCT value was 7.63±1.45, and the CK19 ΔCT value was 6.54±2.23. These results confirmed that PCR molecular detection can determine lymph node metastasis in breast cancer patients by CT value.
PCR detection criteria for SLNs
Adopting an established PCR procedure, PCR detection and histopathological examination were performed on SLNs in 83 breast cancer patients. Of these 83 patients, 53 had negative lymph nodes and 30 had positive lymph nodes. The CT values were analyzed. The mammaglobin CT value of the negative lymph nodes was 28.76±1.44, and the CK19 CT value was 29.02±1.54. The mammaglobin CT value of the positive lymph nodes was 27.20±1.63, and the CK19 CT value was 25.17±0.90. In an analysis of the ΔCT value, the mammaglobin ΔCT value of the negative lymph nodes was 6.35±1.13, and the CK19 ΔCT value was 6.63±1.67. The mammaglobin ΔCT value of the positive lymph nodes was 3.98±1.45, and the CK19 ΔCT value was 2.34±0.79. The rules for the calculation of the RT-PCR CT values for mammaglobin and CK19 in SLNs fresh breast cancer are shown in Figure 3.

The following interpretation criteria were used to determine whether the sentinel lymph node metastasis of breast cancer occurs:
- The average value of the internal reference is between 20–29 cycles;
- If the difference between the CT values of mammaglobin and the CT values of CK19 and the CT values of the internal reference (the ΔCT values) are all greater than 4, the lymph node is negative; however, if 1 is less than 4, there may be metastasis of the lymph nodes.
Clinical significance of PCR molecular detection
A total of 256 SLNs were detected by PCR, and the histopathological and immunohistochemical results were used as the gold standard to verify the feasibility of the molecular detection. Two of the results were judged to be invalid, and their internal parameters did not fall within the specified range. This may have been due to poor tissue processing, excessive fat, or RNA degradation. According to IHC results, among the 254 effective SLNs, 27 were macro-metastatic lymph nodes, 6 were micro-metastatic lymph nodes, 8 were ITC lymph nodes, and 213 were negative lymph nodes. Twenty-three lymph nodes were positive for both tests, and 195 lymph nodes were negative. IHC tests were positive, and 8 lymph nodes were not detected by PCR. Five of these 8 lymph nodes were ITC clusters. The immunohistochemical assay was negative, and 18 PCR results were positive (Figures 4,5).
Statistical analysis
The following calculations were performed to judge the effectiveness of this method:
(TP, true positive; TN, true negative; FN, false negative; FP, false positive)
There was no statistically significant difference (P>0.05) between the statistical paired four-table chi-square test, and there was no difference between the PCR and IHC results (Table 1).
Table 1
| RT-PCR | IHC | χ2 | P | ||
|---|---|---|---|---|---|
| Positive | Negative | Total | |||
| Positive | 33 | 18 | 51 | 3.12 | 0.076 |
| Negative | 8 | 195 | 203 | ||
| Total | 41 | 213 | 254 | ||
SLN, sentinel lymph node; RT-PCR, reverse transcription-polymerase chain reaction; IHC, immunohistochemistry.
Comparative PCR detection and intraoperative frozen detection
The study analyzed 256 sentinel lymph nodes from 150 patients with breast cancer. Taking the results of immunohistochemistry as the gold standard, 41 lymph nodes were positive by immunohistochemistry, of which 8 lymph node metastases were less than 0.2 mm, but freezing during operation was not detected. The metastases in 33 lymph nodes were larger than 0.2 mm, and the metastases of 6 lymph nodes were not detected by freezing during operation, and the metastases of 3 lymph nodes were not detected by PCR method. The detection rate of positive lymph nodes was 65.85% during surgery, and the rate of diagnosis was 94.49%. Statistical chi-square analysis showed that there was a statistically significant difference between intraoperative freezing and immunohistochemical detection results (P<0.05) (Table 2). There was a statistically significant difference between intraoperative freezing and PCR detection (P<0.05) (Table 3). PCR detection is more sensitive than intraoperative frozen detection, and PCR detection is closer to the results of immunohistochemistry.
Table 2
| FS | IHC | χ2 | P | ||
|---|---|---|---|---|---|
| Positive | Negative | Total | |||
| Positive | 27 | 0 | 27 | 16.07 | 0.000* |
| Negative | 14 | 213 | 227 | ||
| Total | 41 | 213 | 254 | ||
*, P<0.01. SLN, sentinel lymph node; FS, frozen slice; IHC, immunohistochemistry.
Table 3
| RT-PCR | FS | χ2 | P | ||
|---|---|---|---|---|---|
| Positive | Negative | Total | |||
| Positive | 25 | 26 | 51 | 18.89 | 0.000* |
| Negative | 2 | 201 | 203 | ||
| Total | 27 | 227 | 254 | ||
*, P<0.01. RT-PCR, reverse transcription-polymerase chain reaction; FS, frozen slice.
Discussion
The incidence of malignant tumors in women in China is the highest in breast cancer patients. Metastasis is an important biological characteristic of malignant tumors, and it is also the fundamental cause of clinical intractability. The question of how to diagnose metastasis in time and to provide proper treatment is the focus, and a difficult area, of clinical research. The status of SLN is an important index for evaluating the prognosis of breast cancer patients. It also has guiding significance in the treatment of local axillae, and can accurately assess the pathological state of axillary lymph nodes (7). As the metastasis of axillary lymph nodes directly affects the 10-year recurrence rate and survival rate of breast cancer patients, axillary lymph nodes are an important indicator of prognosis. The use of SLN biopsy technology in breast cancer patients can reduce surgical trauma, promote the postoperative recovery of upper limb function, and improve patients’ quality of life postoperatively. Axillary lymph node dissection (ALND) can be safely and effectively used in patients with negative axillary lymph nodes to significantly reduce complications and improve quality of life (8). If ALND is performed in patients without axillary lymph node metastasis, the immune barrier of the axillary lymph nodes will be disrupted, and the more easily concealed lesions will be transferred to distant sites (9).
In this study, 256 SLNs from 150 patients were examined by RT-PCR molecular detection to perform a prospective clinical feasibility study. The results of these patients were compared with postoperative permanent histopathology results to verify the potential clinical application of RT-PCR molecular detection. Thirty-three lymph nodes tested positive, and 195 lymph nodes tested negative. Eight metastatic lymph nodes were not detected by RT-PCR; however, the IHC results for these same lymph nodes were positive. The RT-PCR results indicated 18 positive cases; however, the IHC results for these cases were negative. Five of the lymph nodes were diagnosed as isolated tumor cells. The role of isolating tumor cell lymph nodes under the guidelines is not yet clear; however, the metastasis <0.2 mm may not significantly affect the prognosis of the patient (10).
The IHC results were negative; however, the RT-PCR results indicated 18 positive cases. The histological evaluation of HE and IHC sections is considered the “gold standard”, however, IHC diagnostic tests cannot be performed during surgery, which has limitations and is not 100% accurate. Due to practical tissue sampling limitations, the proportion of lymph nodes actually observed by the microscope is less than 5%, and to some extent, the precise assessment of slides also depends on each individual pathologist’s experience and skill. Due to the lack of any comprehensive assessment of lymph node metastasis, it is estimated that 10–15% of clinically relevant metastases are missed in histopathological diagnoses (11,12). Additionally, false-negative and false-positive results in the analysis process produce inconsistencies between RT-PCR and histopathology results. As traditional histology has inherent sampling errors, inconsistencies in the results also appear between diagnosis (traditional histopathology) and slicing (Figure 1B). In relation to Case 1, the RT-PCR and pathological histology results were consistent. In relation to Case 2, the histopathology results did not detect metastases; however, the RT-PCR detection resulted in false-positive results (the actual results were true positive). The situation was the same for Case 3 as Case 2. In relation to Case 4, the histopathology results detected metastases, but the RT-PCR results did not, which resulted in false-negative results (the actual results were true positive). Therefore, RT-PCR detection showed good consistency for larger lymph node metastases.
RT-PCR molecular detection requires the homogenization of SLNs in breast cancer patients; that is, 50% of the submitted lymph nodes homogenize all the nodule masses, and mRNA is extracted and purified from tissue homogenates to minimize the false-negative rate and the false-positive rate. RT-PCR was performed after the RNA is obtained. The real-time RT-PCR assay uses a 1-step reaction to examine the expression of 4 marker genes; that is mammaglobin, CK19, and 2 reference genes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH, and ACTB). Positive and negative quality control checks were performed for each run. The RT-PCR results were only considered valid if the internal and external control results were valid. If one or more SLNs were positive, the patient was considered positive for the BLN test, and if all SLNs were negative, the patient was considered negative for the BLN test.
In relation to the selection of primers, Mitas et al. (13) showed that mammaglobin, breast tissue specificity genes prolactin-inducible protein (PIP), CK19, mammaglobin B, mucin MUC1, and carcinoembryonic antigen (CEA) can be used to detect metastasis in the SLNs of breast cancer patients. Verbanac et al. (14) demonstrated that mammaglobin has a high specificity (99%) and sensitivity (97%) in breast primary tumors. Manzotti et al. (15) detected SLN metastasis in breast cancer patients by detecting the expression of CK19, mammaglobin, CEA, and MUC1 in the SLNs of breast cancer patients. The study showed mammaglobin had the highest sensitivity (77.8%) and specificity. Gimbergues et al. (16) showed that the sensitivity and specificity of mammaglobin were 100% after detecting the expression of mammaglobin, CEA and CK19 in the SLNs of breast cancer patients; thus, mammaglobin is the most accurate marker for diagnosing SLN metastasis. Nissan et al. (17) examined CK19, breast cancer differentiation tumor antigen NY-BR-1, and the mammaglobin B gene for SLN detection, and found that breast cancer differentiation tumor antigen NY-BR-1 sensitivity was higher than that of mammaglobin B. There was an inconsistency in the expression between patients, and the study simultaneously confirmed the above RT-PCR results using deoxyribonucleic acid (DNA) imprinting. Thus, at present, CK19 and mammaglobin B can be used to detect breast cancer SLN metastasis; that is, mammaglobin and CK19 are ideal molecular markers for the detection of SLN metastasis. The RT-PCR molecular assay used in the present study detects the presence or absence of metastasis in SLNs by quantitatively measuring the expression of human mammaglobin and CK19 in freshly homogenized lymph node tissue. Both of these molecular markers are found in breast-derived tumor tissues (18).
In relation to the molecular detection of SLNs in breast cancer patients, a comparison was made between this molecular detection method and the permanent histopathological examination method recommended by the American Society of Clinical Oncology/American Pathologist guidelines. In a study in which the GeneSearch molecular detection method was applied in China (18), the results showed that the overall performance of BLN detection and permanent histopathological examination had a sensitivity of 83.9% (26/31) and a specificity of 95.5% (63/66), respectively. It also had a predicted value of 89.7% (26/29), a negative predictive value of 92.6% (63/68), and an overall agreement of 91.8% (89/97).
The overall consistency of BLN molecular detection in this study was 89.76% (228 results for 254 lymph nodes). The sensitivity and specificity of this method are still somewhat different to those of the US GeneSearch molecular detection method. Eight lymph nodes were diagnosed as metastasis by immunohistochemistry, but were not diagnosed by PCR, and five of them were isolated tumor cells. Molecular detection was still insufficient for metastases less than 0.2 mm. However, through statistical analysis, the difference between PCR method and histopathological results was not statistically significant Therefore, the sensitivity and specificity of PCR in detecting lymph node metastasis are similar to those of paraffin section histopathology.
The two most widely used histological methods for the intraoperative diagnosis of SLNs are frozen sections analyses and imprinted cytology. Previous studies have shown that the sensitivity of frozen section analysis is low, around 55–75% (19,20). Conversely, sentinel lymph node molecular detection has been shown to have a consistently high sensitivity when different experimental methods are used (21).
Compared with IHC, due to the high specificity (91.55%) and NPV (96.06%) of BLN molecular detection, unnecessary axillary dissection or adjuvant treatment can be avoided when BLN molecular detection is applied to SLN examinations. In addition, 5 of the 8 metastases (tumor cell clusters and ITCs) were found to be negative when BLN molecular detection was used. Thus, this assay is more appropriate than IHC for the detection of metastases >0.2 mm. Additionally, the specificity and negative predictive value of BLN molecular detection clearly demonstrate its reliability in guiding ALND.
Conclusions
The results of the RT-PCR in detecting SLN metastases >0.2 mm were similar to the histology results. RT-PCR had objective and rapid output advantages, and was proven to be true and reliable. RT-PCR detection can not only rapidly assess sentinel lymph node status in breast cancer patients during surgery, but its accuracy is also close to that of IHC. Correctly determine whether to perform axillary lymph node dissection and improve the survival rate of patients. The high sensitivity, accuracy, and NPV of the BLN molecular detection compared to IHC detection suggest that BLN detection by 1-step RT-PCR is a viable alternative for assessing SLN metastasis, which has clinical applications. However, the large number of follow-up studies need to be conducted.
Acknowledgments
We are indebted to all the pathologists, breast surgeons and patients.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-485/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-485/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-485/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2018HEC153), and complied with the Declaration of Helsinki (as revised in 2013). All patients signed the informed consent at admission.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Chen W, Wang C, Fu F, et al. A Model to Predict the Risk of Lymph Node Metastasis in Breast Cancer Based on Clinicopathological Characteristics. Cancer Manag Res 2020;12:10439-47. [Crossref] [PubMed]
- Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [Crossref] [PubMed]
- Inoue T, Nishi T, Nakano Y, et al. Axillary lymph node recurrence after sentinel lymph node biopsy performed using a combination of indocyanine green fluorescence and the blue dye method in early breast cancer. Breast Cancer 2016;23:295-300. [Crossref] [PubMed]
- Naidoo K, Pinder SE. Micro- and macro-metastasis in the axillary lymph node A review. Surgeon 2017;15:76-82. [Crossref] [PubMed]
- Monsalve-Lancheros A, Ibáñez-Pinilla M, Ramírez-Clavijo S. Detection of mammagloblin by RT-PCR as a biomarker for lymph node metastasis in breast cancer patients: A systematic review and meta-analysis. PLoS One 2019;14:e0216989. [Crossref] [PubMed]
- Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2014;32:1365-83. [Crossref] [PubMed]
- Baranzelli MC, Penault-Llorca F, Revillon F, et al. Intraoperative determination of axillary node metastasis by RT-PCR. Bull Cancer 2010;97:349-55. [Crossref] [PubMed]
- Soran A, Menekse E, Girgis M, et al. Breast cancer-related lymphedema after axillary lymph node dissection: does early postoperative prediction model work? Support Care Cancer 2016;24:1413-9. [Crossref] [PubMed]
- Martin Martinez MD, Veys I, Majjaj S, et al. Clinical validation of a molecular assay for intra-operative detection of metastases in breast sentinel lymph nodes. Eur J Surg Oncol 2009;35:387-92. [Crossref] [PubMed]
- Orzalesi L, Casella D, Criscenti V, et al. Microinvasive breast cancer: pathological parameters, cancer subtypes distribution, and correlation with axillary lymph nodes invasion. Results of a large single-institution series. Breast Cancer 2016;23:640-8. [Crossref] [PubMed]
- Cserni G. What is a positive sentinel lymph node in a breast cancer patient? A practical approach. Breast 2007;16:152-60. [Crossref] [PubMed]
- Mitas M, Mikhitarian K, Walters C, et al. Quantitative real-time RT-PCR detection of breast cancer micrometastasis using a multigene marker panel. Int J Cancer 2001;93:162-71. [Crossref] [PubMed]
- Verbanac KM, Min CJ, Mannie AE, et al. Long-term follow-up study of a prospective multicenter sentinel node trial: molecular detection of breast cancer sentinel node metastases. Ann Surg Oncol 2010;17:368-77. [Crossref] [PubMed]
- Manzotti M, Dell'Orto P, Maisonneuve P, et al. Reverse transcription-polymerase chain reaction assay for multiple mRNA markers in the detection of breast cancer metastases in sentinel lymph nodes. Int J Cancer 2001;95:307-12. [Crossref] [PubMed]
- Gimbergues P, Dauplat MM, Cayre A, et al. Correlation between molecular metastases in sentinel lymph nodes of breast cancer patients and St Gallen risk category. Eur J Surg Oncol 2007;33:16-22. [Crossref] [PubMed]
- Nissan A, Jager D, Roystacher M, et al. Multimarker RT-PCR assay for the detection of minimal residual disease in sentinel lymph nodes of breast cancer patients. Br J Cancer 2006;94:681-5. [Crossref] [PubMed]
- Liu YH, Xu FP, Liao N, et al. Efficacy of intraoperative GeneSearch Breast Lymph Node (BLN) Assay for breast cancer metastasis detection in sentinel lymph node in Chinese patients. Cancer Sci 2010;101:1920-4. [Crossref] [PubMed]
- Russo L, Betancourt L, Romero G, et al. Frozen section evaluation of sentinel lymph nodes in breast carcinoma: a retrospective analysis. Ecancermedicalscience 2017;11:774. [Crossref] [PubMed]
- Horvath JW, Barnett GE, Jimenez RE, et al. Comparison of intraoperative frozen section analysis for sentinel lymph node biopsy during breast cancer surgery for invasive lobular carcinoma and invasive ductal carcinoma. World J Surg Oncol 2009;7:34. [Crossref] [PubMed]
- Babar M, Madani R, Thwaites L, et al. A differential intra-operative molecular biological test for the detection of sentinel lymph node metastases in breast carcinoma. An extended experience from the first U.K. centre routinely offering the service in clinical practice. Eur J Surg Oncol 2014;40:282-8. [Crossref] [PubMed]


