
Hybrid breast augmentation: our surgical approach and formula for preoperative assessment of fat graft volume
Introduction
Since the first breast augmentation (BA) was performed in 1895 by V. Czerny and the first saline implant was introduced in 1964 by H. G. Arion, many authors described their own techniques (1-5). Nowadays prosthetic materials can be placed in the subglandular (SG), subfascial (SF), submuscular (SM) or dual plane (DP). Each one of these techniques has advantages, disadvantages and specific indications. The SF plane technique was introduced in the 1990s (6,7), as an alternative to the SG plane technique, offering an additional coverage for the implants (6-20). This technique provides a shorter recovery time than the SM plane (6-9,12-14,16,17,19,20).
Contemporary, in the last years autogenous fat grafting (AFG) popularity has increased and the combination between this procedure and the BA gave life to the so-called “composite breast augmentation” (21,22). In fact, in 2009 the American Society of Plastic Surgeons Fat Graft Task Force stated that “Fat grafting may be considered for BA and correction of defects associated with medical conditions and previous breast surgeries” (23). Nowadays this technique changed its name in “hybrid breast augmentation” (HBA). This combination improves the shape of the breast offering a more natural transition between the implant and the mammal gland (19,21,22,24-27).
One of the most relevant challenges of this technique is to assess both the correct place to inject the AFG in and the exact amount of fat that needs to be harvested in order to obtain a desirable aesthetic result and a patients’ high grade of satisfaction. Also, being an aesthetic surgery, it is very important to minimize the percentage of complications. As a matter of fact, plastic surgeons usually calculate the ideal volume of AFG relying mostly on their personal knowledge based on similar previous cases: improper assessment of this amount could hesitate in erroneous evaluations of donor sites and even needless fat transfer. Several researches have analyzed the options of donor areas, AFG preparing, and grafting techniques (28-30). So far only Maximiliano et al. (31) have provided a mathematical equation for assessing the volume of fat to be injected in combined with BA.
In this paper, we describe our surgical technique and mathematical formula in order to assess the proper quantity of fat to be injected and analyzed patients’ grade of satisfaction through Breast-Q© questionnaire. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-21-896/rc).
Methods
Clinical study
A retrospective study of primary and secondary HBA operations was executed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. Ethical approval was unnecessary according to the Italian legislation concerning the guidelines on the observational studies.
All surgeries were performed by the same surgeon over a period of approximately 1 year (Jul 2017–Sep 2018). All participants underwent HBA. We collected data about their age, body mass index (BMI), incisions and about implants: surface, shape, volume and surgical plane. Follow-up for analysis was fixed at less than 15 days, 1, 3, 6 and 12 months.
Preoperative photographs were obtained and compared with postoperative images at follow-up appointments.
Preoperative Breast-Q© was administered to every patient before the surgery and postoperative Breast-Q© was administered at 12 months after surgery in order to evaluate and grade their level of satisfaction with the aesthetic results.
Statistical analysis
A statistical analysis was performed comparing preoperative Breast-Q© with postoperative Breast-Q©: means for every single part of the questionnaires were collected and compared with t-student test.
Relying on Breast-Q© results, we globally defined the patients as follows: “very dissatisfied” patients with a total score between 0 and 25; “somewhat dissatisfied” patients with a total score between 26 and 50; “somewhat satisfied” patients with a total score between 51 and 75; “very satisfied” patients with a total score between 76 and 100.
Preoperative markings/implants selection
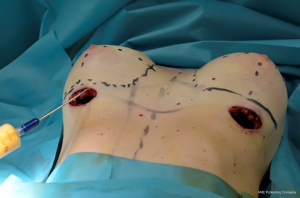
Before surgical procedure, the following marks were drawn with the patient standing in front of the surgeon: midsternal line (MSL), breast meridian (BM), nipple to inframammary fold (N-IMF), inframammary fold (IMF). Two horizontal lines, which represent the inferior and superior limit of the pocket, were marked on the future IMF (FIMF) and the superior breast line (SBL) in relation with the length and height of the chosen implants. The medial and lateral limits of the pocket were drawn as two vertical lines starting from the base of the pocket, according to the dimension of the implants. The circumferential limits of the glands were obtained making the patient lay down. In the end, an inter-nipple line (INL) which continued to the lateral limit of the pocket was drawn in order to divide the breasts in 4 quadrants where the AFG was injected. We consider these markings to be the best choice in order to obtain a precise centering of the prosthesis, to maintain accurate pocket sized related to the implant volume and to inject the fat properly in the 4 quadrants.
The implant volume has been chosen together with the patients, considering their height, weight and thoracic cage diameter, trying to match these measures with their wish and expectations but also identifying and aiming to correct breast and thoracic asymmetries.
Surgical technique and AFG
All the participants of this research received high- or low-profile implants in the SF plane. All procedures were performed through a 4 cm incision made 1 cm laterally along the vertical inferior projection of the medial margin of the areola on the IMF. Tumescent local anesthesia was performed on every patient: the tumescent solution was prepared with 25 mL of 2% lidocaine, 8 mEq of sodium bicarbonate, and 1 mL of epinephrine (1 mg/1 mL) in 1,000 mL of 0.9% saline solution (32). 500 mL were injected in every single zone.
Fat graft was harvested from abdomen, trochanteric regions and back using a 4-mm cannula with beveled 0.5-mm ports connected to the PureGraft© System 850 mL (Figure 1) and to the suction system LipoSurg©. The AFG was purified and filtered using lactated Ringer solution through the closed system and transferred to 60-mL Luer-Lock syringes (Figure 2) for injection. According to Coleman principles (33), a 50% of the AFG is injected, through the IMF incision, in the suprafascial plane below the mammary gland throughout a 15-cm cannula with diameters from 1.9 to 2.1 mm, with retrograde strings starting from the upper poles of the breast towards the inferior ones (Figure 3). The other 50% of the AFG is then injected superficially and radially in the subcutaneous above the mammary gland tissue around the 4 quadrants, through 4 incisions performed at h12, h3, h6 and h9 around the areola with a 11-caliber blade (Figure 4). The phases of the procedure are shown in Figure 5.

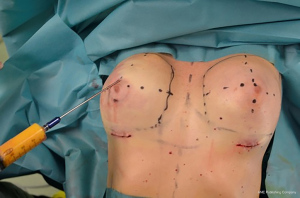
No suction drains were used and a mild compression with elastic bands was placed over the upper breast poles.
All patients were given intravenous antibiotic prophylaxis with Cefazolin or Clindamycin in case of allergy. All the patients were discharged five hours after the surgery. The elastic band was maintained for 4 weeks; patients were invited to massage and mobilize their breasts after 1 month. Physical activities were avoided for 5 weeks.
Mathematical equation
So as to attain the desired contour and outcome, the implant volume was chosen together with the patients, considering their height, weight and thoracic cage diameter, trying to match our goals with their wish and expectations but also identifying and aiming to correct breast and thoracic asymmetries. In accordance with the principle that an increase in each size corresponds to 130 mL (34), we firstly decided the final volume of the breasts (VF) which is the sum of the initial breast volume (VB) and the augmentation volume (VA).
We believe that the ideal VA is reached combining half through implant and a half through AFG (Fv).
In our experience, the harvested fat (FH) must be the double of the final FV, because an average 50% of the harvest is reabsorbed (Fr) and only the remaining 50% remains in the injection site (FR). In fact, we noticed that, through the PureGraft© System, half of the injected fat is reabsorbed. So we have that:
These basic calculations yield the mathematical formula for calculating the composition of the VA:
For example: a patient has A-sized breasts (VB) and wants to reach C-sized breasts (VF). In order to obtain this result, a two-sizes increment (VA) is necessary. This increment is quantified in 260 mL. We chose a 130 mL implant [IV(50%)] and harvested and injected 260 mL of fat (FH). After one month, approximately 130 mL of fat are reabsorbed (Fr) and only 130 mL remains in the injection site (FR). In this simple way we obtained our two sizes increment (260 mL).
Results
The authors executed this surgical procedure in 122 patients (244 breasts): 75 (61.47%) primary HBA and 47 (38.53%) secondary HBA. The implants used in this study were all Ergonomix-style Motiva Smooth/SilkSurface with high/low-projection and their average volume was 170 mL (range, 120 to 225 mL). The demographic data and mean anthropomorphic measurements are shown in Table 1. Mean age was 43 years (range, 19 to 65 years), mean BMI 26 kg/m2 (range, 21 to 30 kg/m2) and mean pinch test 1.4 cm (range, 1.0 to 1.9 cm). The mean volume of harvested and grafted fat was 600 mL (range, 480 to 720 mL) and 150 mL per breast (range, 120 to 180 mL). AFG was harvested in 76% of cases from the trochanteric regions and the back and in 24% of cases from the abdomen, trochanteric regions and back. Mean operating time was 80 minutes (range, 60 to 120 minutes).
Table 1
| Characteristic | Mean | Range |
|---|---|---|
| Age (years) | 43 | 19–65 |
| BMI (kg/m2) | 26 | 21–30 |
| SN-N (cm) | 19.4 | 18–23 |
| N-IMF (cm) | 6.7 | 5.1–7.7 |
| IM (cm) | 4.2 | 2.0–5.1 |
| Volume of implants (mL) | 170 | 120–225 |
| Total AFG harvest volume (mL) | 600 | 480–720 |
| AFG grafted volume per breast (mL) | 150 | 120–180 |
BMI, body mass index; SN-N, sternal notch-to-nipple distance; N-IMF, nipple to inframammary fold; IM, intermammary distance; AFG, autologous fat graft.
Outcomes/complications
Breast-Q© questionnaires analysis showed (see Table 2): for the “Psychosocial well-being” module a preoperative mean of 27.09 (range, 0–44) and a postoperative one of 89.67 (range, 89–100) (P=7.71-128), for the “Sexual well-being” module a preoperative mean of 24.98 (range, 13–51) and a postoperative one of 91.17 (range, 84–100) (P=1.93-106), for the “Satisfaction with breasts” module a preoperative mean of 26.92 (range, 0–47) and a postoperative one of 92.28 (range, 91–100) (P=3.19-106), for the “Psychosocial well-being: chest” module a preoperative mean of 20.87 (range, 10–38) and a postoperative one of 91.73 (range, 84–100) (P=2.58-111), for the “Satisfaction with implants” module a postoperative mean of 7.68 (range, 6–8), for the “Satisfaction with outcomes” module a postoperative mean of 94.41 (range, 89–100).
Table 2
| Module | Preop. mean [range] | Postop. mean [range] | P value t-student test |
|---|---|---|---|
| Psychosocial well-being | 27.09 [0–44] | 89.67 [89–100] | 7.7128E-128 |
| Sexual well-being | 24.98 [13–51] | 91.17 [84–100] | 1.9289E-106 |
| Satisfaction with breasts | 26.92 [0–47] | 92.28 [91–100] | 3.1934E-106 |
| Psychosocial well-being: chest | 20.87 [10–38] | 91.73 [84–100] | 2.5769E-111 |
| Satisfaction with implants | – | 7.68 [6–8] | – |
| Satisfaction with outcome | – | 94.41 [89–100] | – |
One hundred and twenty-two (100%) patients were globally “very satisfied” with their results and did not regret undergoing the HBA surgery during a 12-month follow-up.
Thirteen cases of complications presented in 12 patients (10.65%): hypertrophic scarring in 5 cases (4.09%), wound dehiscence in 3 cases (2.46%), 3 cases of hematomas (2.46%), 1 case of seroma (0.82%), 1 case of fat necrosis (0.82%). No infections were reported. Bilateral hypertrophic scarring was observed in 1 patient (0.82%), while monolateral hypertrophic scarring presented in 3 cases (2.46%) (Table 3). Three patients (2.46%) previously underwent breast augmentation with Macrolane injection (35). All the patients were instructed to massage their scars with KaralisÒ and medicated with polyurethane dressing with ibuprofen (36). When the follow-up was completed after 1 year, all the patients were satisfied with the result and did not want to undergo surgical scar revision. The 3 dehiscence wound cases did not develop pathological scars and the final outcome was satisfactory.
Table 3
| Complication | Number | % |
|---|---|---|
| Hypertrophic scarring | 5 | 4.09 |
| Wound dehiscence | 3 | 2.46 |
| Hematoma | 3 | 2.46 |
| Seroma | 1 | 0.82 |
| Fat necrosis | 1 | 0.82 |
| Infection | 0 | 0 |
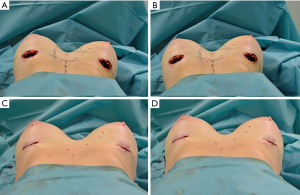
Hematomas and seromas were drained with success in a single outpatient treatment (Figures 6-8).
Discussion
In our study, we inserted the implants in the SF plane because of its well-known satisfactory outcomes (6-20) and advantages which include lower morbidity and the capability to give more soft tissue coverage of the prosthetic materials than the SG plane (7,8,12-14,20).
One of the most relevant issues of this procedure is insufficient soft tissue coverage in patients with a very low BMI (19). The average BMI of our court of patients was 26 (range, 21–30) kg/m2. If breast tissue thickness is less than 20 mm (real coverage 5–10 mm), the coverage offered by the pectoral fascia is not sufficient, and a SM plane is suggested. In these situations, SF HBA may represent a valid option to camouflage the prosthesis similarly to the SM plane (19,21,22,24-27,31).
The reduced thickness of the glandular and subcutaneous tissue can be evident especially in the upper medial and lateral poles; in these scenarios an AFG can refine and meliorate the passage area among pectoralis muscle, sternum and prosthesis (Figures 6-8) (19,21,22,24-27).
In fact, in the last years, AFG and HBA popularity has increased and became more attractive and frequent in patients with low coverage (19,21,22,24-27).
Nowadays, AFG is largely used as an adjuvant in plastic surgery to recondition volume and profile defects with differences on the phases of the procedure: harvesting, preparation, and grafting (28-30). Moreover, latest improvements of the AFG technique have allowed diminutions in the incidence of well-known complications as reabsorption, lumps, and fat necrosis (19,21,22,24-30,33,37-39).
Although a proper evaluation of the AFG is essential to HBA procedures, the plane which the fat must be injected into and the exact volume have not been codified yet. Many plastic surgeons inject the fat only into the subcutaneous tissue and estimate the ideal AFG volume relying on their personal experience based on similar previous cases they have dealt with. These abstract methods in some cases lead to an underestimated volume which causes the necessity of an ulterior surgical time for fat harvesting, in other cases the volume is overestimated with consequent dissatisfaction of the patients, unnecessary procedures and wasted fat. In addition, erroneous estimations of AFG volume may lead to inexact evaluation of donor sites, and even prevent eventual further operations if extra grafts are necessary.
Contrary to Maximiliano et al. (31), we injected the AFG not only in the upper quadrants of the breast but also in the inferior ones in both the suprafascial and SC planes, surrounding the mammary gland. In our opinion, a double layer of fat represents a good solution in order to reduce the absorption of fat and offer a proper coverage to the underlying implant. The deep layer is injected in the suprafascial plane from the upper poles toward the inferior ones and the superficial layer is injected into the subcutaneous tissue radially around the 4 quadrants. This double layer approach has not been described in literature yet.
Lately, silicone implants faced the development of new superficies and viscoelastic gel properties (40-43). With advances in gel technology, the Ergonomix-style Motiva Smooth/SilkSurface prosthesis are characterized by a very low roughness for the purpose of prevent tissue outgrowth and reduce bacteria adhesion (40-43). In addition to the peculiarities of the surface, these implants integrate intensified rheologic properties, mimicking the native dynamic forces of the mammary gland (42). Ergonomix implants incorporate ProgressiveGel Ultima, a highly elastic gel with low viscosity and superior adaptability capabilities that offers a more natural contour (41,42). In our study these implants, with volumes ranging from 120–225 mL (mean, 170 mL), were used.
Having said that, we believe that an ergonomic implant already has the intrinsic property to change its shape according to gravity. Consequently, contrary to Maximiliano et al. (31), we did not use the AFG to avoid a sharp passage from the area with the prosthesis and the gap with the upper pole, due to insufficient coverage. Likewise, we did not use the AFG to simulate the aesthetic outcomes of an anatomic implant or to transform the upper pole of a round implant into a conical one.
Performing HBA, the injection of a consistent percentage of fat allows surgeons to use smaller implants and reduce well known complications related to bigger ones such as sensory alteration of the nipple-areolar complex (44), formation of striae distensae (SD) (45), capsular contraction (46), gland thinning and hematomas. Moreover, implants are less palpable and fat consistency can mimic a natural breast better than implants. By injecting fat into the breasts, we were able to “fill” the atrophic glands, stretch the skin and consequently correct grade 1 and 2 of breast ptosis (Figure 7).
To date, thanks to advances in engineering medical systems, Maximiliano et al. have provided a mathematical equation to predict the volume of fat to be grafted. They introduced a technique based on a mathematical formula, which uses implant base/projection to make a prediction of the necessary AFG to obtain a harmonic passage between areas with and without the prosthesis (31).
In our study, in order to obtain the desired shape and outcome, the implant volume was chosen together the patients, considering their height, weight and thoracic cage diameter, trying to match these measures with their wish and expectations but also identifying and aiming to correct breast and thoracic asymmetries. In accordance with the principle that an increase in each size corresponds to 130 mL (34), we firstly decided the final volume of the breasts. Then we applied our mathematical formula in order to obtain the best possible aesthetic result.
In our sample the range of age was from 19 to 65 years (mean, 43 years) and BMI from 21 to 30 kg/m2 (mean, 26 kg/m2) with very different quality of skin and fat. In these heterogeneous group we observed a first aesthetical result at 3 months, the implants reached their final position at 6 months and we observed the stability of the outcomes at 1 year.
In our study, the same operator used the above-mentioned technique in 122 patients (between Jul 2017 and Sep 2018) avoiding inter-operator variability. Information and results associated with the surgeries were gathered and retrospectively analyzed. Follow-up for analysis was fixed at less than 15 days, 1, 3, 6 and 12 months. Outcome and satisfaction were analyzed, through a validated PRO such Breast-Q©, at 12 months to evaluate correct AFG implantation and full resolve swelling and edema related to surgery. One hundred and twenty-two patients (100%) were very satisfied with their results. A satisfying aesthetic outcome was acquired, preserving a harmonic breast contour and regular transition from the upper poles to the implant site. The grafted volume was large (mean, 150 mL per breast; range, 480–720 mL), and the implants volume was small/medium (mean, 170 mL; range, 120–225 mL), which, in combination with good grafting technique, stimulates AFG implantation.
In spite of the aesthetic benefits, surgeries combining AFG and breast prosthesis involve some disadvantages such as the necessity of former practice and specific surgical abilities combined with supplementary costs and a longer operative time which represents a moderate drawback. As a matter of fact, with a proper training the additional operative time should diminish (31). In fact, in our research, the mean operating time was 80 minutes (range, 60–120 minutes) and liposuction was limited and performed exclusively to collect fat for the hybrid surgery. Complications observed were minor: hypertrophic scarring, wound dehiscence, hypertrophic scarring, seroma, hematoma, fat necrosis. No infections were reported (Table 2).
Our study has some limitations. First, it is an observational and nonrandomized study, and may consequently have been biased. We continue collecting prospective records regarding this technique so as to increase our cases and describe eventual results. Second, only one model and style of Motiva SmoothSilk/SilkSurface implants (Ergonomix, high projection) was used and our equation was used to implants with volumes ranging from 120 to 225 mL. Ulterior investigations are needed to estimate if the results apply to implants other than the studied group in the current research and for different prosthesis styles (such as saline-filled, nondynamic gel implants and Ergonomix with different projection styles).
Progress in AFG techniques and new-generation silicone gel prosthesis have driven to relevant advancement in aesthetic results following BA (31). Our technique may take a meaningful part in BA, and the outcomes of our papers demonstrate it to be a manageable and predictable operation, offering optimal aesthetic results with a harmonic contour, and adequate size and projection.
Conclusions
Our surgical technique, described in this paper, showed a low rate of complications and reduced operative and recovery times. Our mathematical formula, used to calculate the volume of fat that needs to be injected, seems to be validly predictive and a precise guide for surgical decision-making in planning the treatment of thin patients who are candidated for HBA because of hypomastia. In fact, globally, the analysis of Breast-Q© questionnaires showed a high grade of satisfaction among patients, proving the validity both of our surgical technique and of our formula.
Further investigations should be performed in order to study a wider population and different type of implants.
Acknowledgments
We thank Dr. Iulia Elena Marin for the linguistic revision.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-21-896/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-896/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-896/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-21-896/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang EI, Hammond DC. Clinical Results on Innovation in Breast Implant Design. Plast Reconstr Surg 2018;142:31S-8S. [Crossref] [PubMed]
- Lista F, Ahmad J. Evidence-based medicine: Augmentation mammaplasty. In: Plastic Surgery Complete: The Clinical Masters of PRS- Breast Augmentation. Wolters Kluwer Health Adis (ESP); 2015:1-14.
- Adams WP, Mallucci P. Breast augmentation. In: Plastic Surgery Complete: The Clinical Masters of PRS- Breast Augmentation. Wolters Kluwer Health Adis (ESP); 2015:15-29.
- Young VL, Watson ME. Breast implant research: where we have been, where we are, where we need to go. Clin Plast Surg 2001;28:451-83. vi. [Crossref] [PubMed]
- Hidalgo DA. Breast augmentation: choosing the optimal incision, implant, and pocket plane. Plast Reconstr Surg 2000;105:2202-16; discussion 2217-8. [Crossref] [PubMed]
- Graf RM, Bernardes A, Auersvald A, et al. Subfascial endoscopic transaxillary augmentation mammaplasty. Aesthetic Plast Surg 2000;24:216-20. [Crossref] [PubMed]
- Góes JC, Landecker A. Optimizing outcomes in breast augmentation: seven years of experience with the subfascial plane. Aesthetic Plast Surg 2003;27:178-84. [Crossref] [PubMed]
- Graf RM, Bernardes A, Rippel R, et al. Subfascial breast implant: a new procedure. Plast Reconstr Surg 2003;111:904-8. [Crossref] [PubMed]
- Stoff-Khalili MA, Scholze R, Morgan WR, et al. Subfascial periareolar augmentation mammaplasty. Plast Reconstr Surg 2004;114:1280-8; discussion 1289-91. [Crossref] [PubMed]
- Ventura OD, Marcello GA. Anatomic and physiologic advantages of totally subfacial breast implants. Aesthetic Plastic Surgery 2005;29:379-83. [Crossref] [PubMed]
- Jinde L, Jianliang S, Xiaoping C, et al. Anatomy and clinical significance of pectoral fascia. Plast Reconstr Surg 2006;118:1557-60. [Crossref] [PubMed]
- Munhoz AM, Fells K, Arruda E, et al. Subfascial transaxillary breast augmentation without endoscopic assistance: technical aspects and outcome. Aesthetic Plast Surg 2006;30:503-12. [Crossref] [PubMed]
- Serra-Renom J, Garrido MF, Yoon T. Augmentation mammaplasty with anatomic soft, cohesive silicone implant using the transaxillary approach at a subfascial level with endoscopic assistance. Plast Reconstr Surg 2005;116:640-5. [Crossref] [PubMed]
- Munhoz AM, Aldrighi C, Ono C, et al. The influence of subfascial transaxillary breast augmentation in axillary lymphatic drainage patterns and sentinel lymph node detection. Ann Plast Surg 2007;58:141-9. [Crossref] [PubMed]
- Benito-Ruiz J, Raigosa M, Manzano M, et al. Subfascial breast augmentation: thickness of the pectoral fascia. Plast Reconstr Surg 2009;123:31e-2e. [Crossref] [PubMed]
- Tijerina VN, Saenz RA, Garcia-Guerrero J. Experience of 1000 cases on subfascial breast augmentation. Aesthetic Plast Surg 2010;34:16-22. [Crossref] [PubMed]
- Hunstad JP, Webb LS. Subfascial breast augmentation: a comprehensive experience. Aesthetic Plast Surg 2010;34:365-73. [Crossref] [PubMed]
- Aygit AC, Basaran K, Mercan ES. Transaxillary totally subfascial breast augmentation with anatomical breast implants: review of 27 cases. Plast Reconstr Surg 2013;131:1149-56. [Crossref] [PubMed]
- Sampaio Goes JC, Munhoz AM, Gemperli R. The Subfascial Approach to Primary and Secondary Breast Augmentation with Autologous Fat Grafting and Form-Stable Implants. Clin Plast Surg 2015;42:551-64. [Crossref] [PubMed]
- Munhoz AM, Gemperli R, Sampaio Goes JC. Transaxillary Subfascial Augmentation Mammaplasty with Anatomic Form-Stable Silicone Implants. Clin Plast Surg 2015;42:565-84. [Crossref] [PubMed]
- Auclair E, Blondeel P, Del Vecchio DA. Composite breast augmentation: soft-tissue planning using implants and fat. Plast Reconstr Surg 2013;132:558-68. [Crossref] [PubMed]
- Auclair E, Anavekar N. Combined Use of Implant and Fat Grafting for Breast Augmentation. Clin Plast Surg 2015;42:307-14. vii. [Crossref] [PubMed]
- Coleman SR, Saboeiro AP. Primary Breast Augmentation with Fat Grafting. Clin Plast Surg 2015;42:301-6. vii. [Crossref] [PubMed]
- Zheng DN, Li QF, Lei H, et al. Autologous fat grafting to the breast for cosmetic enhancement: experience in 66 patients with long-term follow up. J Plast Reconstr Aesthet Surg 2008;61:792-8. [Crossref] [PubMed]
- Bravo FG. Parasternal infiltration composite breast augmentation. Plast Reconstr Surg 2015;135:1010-8. [Crossref] [PubMed]
- Serra-Mestre JM, Fernandez Peñuela R, Foti V, et al. Breast Cleavage Remodeling with Fat Grafting: A Safe Way to Optimize Symmetry and to Reduce Intermammary Distance. Plast Reconstr Surg 2017;140:665e-72e. [Crossref] [PubMed]
- Kerfant N, Henry AS, Hu W, et al. Subfascial Primary Breast Augmentation with Fat Grafting: A Review of 156 Cases. Plast Reconstr Surg 2017;139:1080e-5e. [Crossref] [PubMed]
- Gir P, Brown SA, Oni G, et al. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg 2012;130:249-58. [Crossref] [PubMed]
- Saint-Cyr M, Rojas K, Colohan S, et al. The role of fat grafting in reconstructive and cosmetic breast surgery: a review of the literature. J Reconstr Microsurg 2012;28:99-110. [Crossref] [PubMed]
- Chan CW, McCulley SJ, Macmillan RD. Autologous fat transfer--a review of the literature with a focus on breast cancer surgery. J Plast Reconstr Aesthet Surg 2008;61:1438-48. [Crossref] [PubMed]
- Maximiliano J, Munhoz AM, Pedron M, et al. Hybrid Breast Augmentation: A Reliable Formula for Preoperative Assessment of Fat Graft Volume Based on Implant Volume and Projection. Aesthet Surg J 2020;40:NP438-52. [Crossref] [PubMed]
- Bolletta A, Dessy LA, Fiorot L, et al. Sub-muscular Breast Augmentation Using Tumescent Local Anesthesia. Aesthetic Plast Surg 2019;43:7-13. [Crossref] [PubMed]
- Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg 2001;28:111-9. [Crossref] [PubMed]
- King NM, Lovric V, Parr WCH, et al. What Is the Standard Volume to Increase a Cup Size for Breast Augmentation Surgery? A Novel Three-Dimensional Computed Tomographic Approach. Plast Reconstr Surg 2017;139:1084-9. [Crossref] [PubMed]
- Trignano E, Rusciani A, Armenti AF, et al. Augmentation Mammaplasty After Breast Enhancement With Hyaluronic Acid. Aesthet Surg J 2015;35:NP161-8. [Crossref] [PubMed]
- Cigna E, Tarallo M, Bistoni G, et al. Evaluation of polyurethane dressing with ibuprofen in the management of split-thickness skin graft donor sites. In Vivo 2009;23:983-6. [PubMed]
- Del Vecchio DA. "SIEF"--simultaneous implant exchange with fat: a new option in revision breast implant surgery. Plast Reconstr Surg 2012;130:1187-96. [Crossref] [PubMed]
- Abboud MH, Dibo SA. Immediate Large-Volume Grafting of Autologous Fat to the Breast Following Implant Removal. Aesthet Surg J 2015;35:819-29. [Crossref] [PubMed]
- Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg 2007;119:775-85; discussion 786-7. [Crossref] [PubMed]
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases. Aesthet Surg J 2018;38:S62-73. [Crossref] [PubMed]
- Quirós MC, Bolaños MC, Fassero JJ. Six-Year Prospective Outcomes of Primary Breast Augmentation With Nano Surface Implants. Aesthet Surg J 2019;39:495-508. [Crossref] [PubMed]
- Sforza M, Hammond DC, Botti G, et al. Expert Consensus on the Use of a New Bioengineered, Cell-Friendly, Smooth Surface Breast Implant. Aesthet Surg J 2019;39:S95-S102. [Crossref] [PubMed]
- Huemer GM, Wenny R, Aitzetmüller MM, et al. Motiva Ergonomix Round SilkSurface Silicone Breast Implants: Outcome Analysis of 100 Primary Breast Augmentations over 3 Years and Technical Considerations. Plast Reconstr Surg 2018;141:831e-42e. [Crossref] [PubMed]
- Stuebe AM, Horton BJ, Chetwynd E, et al. Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. J Womens Health (Larchmt) 2014;23:404-12. [Crossref] [PubMed]
- Basile FV, Basile AV, Basile AR. Striae distensae after breast augmentation. Aesthetic Plast Surg 2012;36:894-900. [Crossref] [PubMed]
- Fraldi M, Esposito L, Cutolo A, et al. Stealthy role of size-driven stresses in biomechanics of breast implants capsular contracture. J Mech Behav Biomed Mater 2016;64:199-208. [Crossref] [PubMed]






