
Impact of radiologic splenic vessel invasion in resectable left-sided pancreatic ductal adenocarcinoma: predictor of early systemic recurrence following upfront surgery
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and lethal gastrointestinal malignancies (1). Only around 20% of patients are eligible for initial resection (2). National Comprehensive Center Network (NCCN) guidelines recommend that the standard treatment for potentially resectable PDAC (R-PDAC) is upfront surgery followed by adjuvant therapy to achieve R0 resection and better survival outcomes (3). However, recent randomized clinical trials reported that upfront surgery followed by adjuvant therapy resulted in R0 resection in 40–87%, the median recurrence-free survival (RFS) was 11.3–22.9 months, and the median overall survival (OS) was 25.5–54.4 months (4-6). These survival outcomes indicate that a subset of tumors with a poor prognosis is being included within R-PDAC. Therefore, interest in neoadjuvant therapy (NAT) is increasing even among patients with R-PDAC (7,8).
About 15% of PDAC cases occur in the body and tail of the pancreas (9). Left-sided PDAC, a malignancy originating in the body or tail of the pancreas, is asymptomatic until the disease develops to an advanced stage with metastasis (10). Recently, several retrospective studies have reported that NAT for resectable left-sided PDAC was associated with similar or improved OS in comparison with upfront surgery (11-13). However, in these studies, the effects of NAT differed according to the subset of the study cohort, such as stage and radiologic splenic vessels (SVs) invasion. It is important to properly select patients with resectable left-sided PDAC who are suitable for NAT.
The purpose of this study was to identify prognostic factors and the best candidates for NAT among patients with resectable left-sided PDAC by analyzing the timing and pattern of recurrence following upfront surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-304/rc).
Methods
Patients
All consecutive patients who underwent upfront distal pancreatectomy for resectable left-sided PDAC between January 2005 and December 2015 at a single institution were evaluated. Only patients with a confirmed pathological diagnosis of pancreatic adenocarcinoma were included. Patients diagnosed with other malignancies within 5 years before and after distal pancreatectomy and those with incomplete follow-up were excluded. Finally, we included 311 patients. This study was approved by the Institutional Review Board of Asan Medical Center (Approval No. 2019–1404) and informed consent was waived due to the retrospective study design. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Demographics, clinical and treatment characteristics
The following characteristics were collected for each patient: age at surgery, sex, Charlson age-comorbidity index, body mass index (BMI), waiting time to surgery, preoperative and postoperative serum CA 19-9 and carcinoembryonic antigen (CEA), neutrophil to lymphocyte ratio (NLR), tumor size on preoperative computed tomography (CT), maximum standard uptake value (SUVmax) on preoperative 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), postoperative complications, and adjuvant therapy.
We defined the waiting time to surgery as the duration from the first diagnosis of PDAC to surgery. Tumor size was measured by the maximal cross-sectional diameter of the lesion. Postoperative complications were classified according to the Clavien-Dindo classification (14). A clinically relevant postoperative pancreatic fistula (CR-POPF) was defined according to the revised 2016 ISGPS (International Study Group on Pancreatic Surgery) classification and grading of POPF (15). After surgery, patients were routinely referred to a medical or radiation oncologist for adjuvant treatment recommendations.
Surgical procedure
All included patients underwent open, laparoscopic, or robotic distal pancreatectomy by experienced pancreatic surgeons. En bloc resection of the pancreatic body/tail, spleen, and regional lymph node (LN) were performed. There were no major vascular resections other than SVs. In selected cases, depending on the degree of tumor invasion, both anterior or posterior radical antegrade modular pancreatosplenectomy (16) and combined adjacent organ resection were performed to achieve negative resection margins.
Radiologic image analyses
All included patients underwent contrast-enhanced CT preoperatively. To evaluate the resectability status and determine whether there was radiologic SV invasion, the CT images were retrospectively reviewed by two pancreatic surgeons with more than 15 years of experience and more than 5 years of experience, respectively.
The NCCN guideline (version 2.2017) defines resectability status based on the tumor’s major vascular characteristics (3). We selected patients with resectable left-sided PDAC by analyzing preoperative CT scans according to the NCCN guidelines. SVs invasion was defined as invasion of the splenic artery, splenic vein, or both. Radiologic SVs invasion was defined as follows: (I) tumor abutment with SVs narrowing or deformity of the vessel wall (Figure 1); or (II) SVs encasement or occlusion by the tumor (Figure 2).
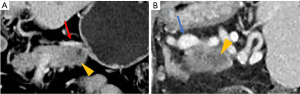
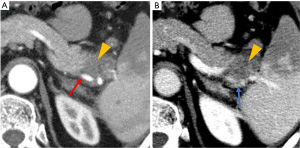
Pathological evaluation
The pathologic reports described the tumor characteristics, including the pancreas transection margin, radial resection margin, tumor size, T-stage, LNs status, N-stage, tumor differentiation grade, and presence of microscopic lymphovascular or perineural invasion. The cancer stage was defined according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system (17). Resection margin (R) status was defined as R0 when the distance from the tumor cells to the closest resection margin was >1 mm or R1 when the distance was ≤1 mm (18). Invasion of the SVs was defined as positive when the tumor cells had clearly infiltrated into the adventitia at a minimum, if not further, into the vessel wall. The institution of present study has been obligated to report the presence or absence of SVs invasion in pathologic reports of specimens following distal pancreatectomy since 2012. In previous reports, the presence of SVs invasion was omitted in some cases.
Adjuvant chemotherapy and radiotherapy
Adjuvant chemotherapy and radiotherapy was determined by the oncologists and radiotherapy-oncologists on the consultation of the pancreatic surgeons. Adjuvant chemotherapy comprising gemcitabine, conventional 5-fluorouracil (5-FU), 5-FU with leucovorin (FL) or capecitabine was administered. Conventional 5-FU chemotherapy consisted of 500 mg/m2 for 5 consecutive days for 4 weeks. FL chemotherapy consisted of leucovorin 25 mg/m2 body surface area (BSA) by 2-hour intravenous infusion, followed by 5-FU 375 mg/m2 by bolus intravenous infusion for 5 consecutive days for 4 weeks. Gemcitabine chemotherapy consisted of six cycles of either 1,000 mg/m2 intravenous gemcitabine administered once a week for three of every 4 weeks. Oral capecitabine chemotherapy administrated 1,250 mg/m² twice daily on days 1–14 of a 21-day cycle, for eight cycles. Three-dimensional conformal radiotherapy consisted of a total dose of 45 Gy (1.8 Gy daily fraction, five fractions per week for 5 weeks), followed by an additional 9 Gy (1.8 Gy daily fraction, five fractions).
Follow-up and recurrence
Patients were followed up by abdominal contrast-enhanced CT scan and tumor markers (CA 19-9, CEA) every 3 months for the first 2 years after primary surgery, and then every 3–6 months thereafter. Recurrence was defined as newly detected, progressive soft tissue or mass like lesions at specific sites, as detected on CT scans during regular follow-up after primary surgery. Occasionally, additional imaging modalities such as magnetic resonance imaging or FDG-PET were performed to determine the pattern of recurrence. When imaging findings were consistent with a recurrence, biopsy was not routinely performed. In the present study, only the first recurrence site was used when analyzing the pattern of recurrence. Local recurrence was defined as a recurrence in the remnant pancreas or in the surgical bed, such as the soft tissue along the celiac or superior mesenteric artery (SMA), or at the splenectomy site. The liver, lungs, abdominal wall, bones, peritoneal seeding and distant LNs such as para-aortic and subclavian LNs, were regarded as distant sites. We defined systemic recurrence as distant recurrence ± local recurrence.
Statistical analysis
Continuous variables are summarized as mean and standard deviation and were compared using Student’s t-test. Categorical variables are presented as counts and percentages and were compared using chi-square tests. RFS was defined as the time from surgery to either the first recurrence or death. OS was defined as the time from surgery to death. Post-recurrence survival (PRS) was defined as the time from recurrence to death. RFS, PRS and OS rates were estimated using the Kaplan-Meier method and the log-rank test was applied to compare the subgroups. A minimum P value approach was used to evaluate the optimal cutoff value of RFS to divide the patients into early and late recurrence groups. The log-rank test was performed for different lengths of RFS to determine the optimal cutoff with the lowest P value. Multivariate analyses were performed using a Cox proportional hazard regression model to identify the predictors for early systemic recurrence. For multivariate analysis, multivariate normal imputations were performed for missing data (19). All tests were two-sided, and P values <0.05 were considered statistically significant. The analyses were performed using SPSS version 24 (SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics with or without recurrence
The median follow-up for the entire cohort was 29.3 months. At the time of the last follow-up, 241 (77.5%) of 311 patients had a recurrence after a median RFS of 11.4 (95% CI: 9.9–12.9) months. The median PRS was 11.4 (95% CI: 10.0–12.8) months. The patients with recurrence were analyzed according to the timing and site of recurrence (Figure 3).
The patients’ characteristics according to the recurrence are shown in Table 1. Of the entire cohorts of 311 patients, 307 patients had preoperative CA 19-9 values available, 257 had SUVmax on preoperative FDG-PET, 302 had pathologic differentiation, 221 had pathologic SV invasion, 308 had postoperative CA 19-9 values, respectively. There were no statistically significant differences in age, sex, BMI, or Charlson-age comorbidity index between the groups. Preoperative CA 19-9 (69.7 vs. 29.9 U/mL, P=0.018) was significantly higher and the tumor size on preoperative CT (2.89 vs. 2.24 cm, P<0.001) was significantly larger in patients with recurrence than in those without recurrence. The radiologic SVs invasion and combined adjacent organ resection were significantly more frequent (13.8% vs. 2.9%, P=0.001) in patients with recurrence than in those without recurrence. Of 174 patients with radiologic SVs invasion, 28 had splenic artery invasion, 55 had splenic vein invasion, and 91 had both. The radiologic SVs invasion status was classified as abutment in 91 patients, and encasement in 83 patients. For the pathologic results, a higher T-stage (T3–4; 28.2% vs. 10.0%, P=0.002), higher N-stage (N1–2; 55.2% vs. 27.1%, P<0.001), lymphovascular invasion (44.4% vs. 24.3%, P=0.002), perineural invasion (82.2% vs. 58.6%, P<0.001), and positive radial resection margin (25.7% vs. 4.3%, P<0.001) were significantly more frequent in patients with recurrence than in those without a recurrence. There were no statistically significant differences in postoperative CA 19-9, clinically relevant POPF or postoperative complications between the groups. Median interval between surgery and adjuvant chemotherapy for the entire cohort was 36.0 days. There was no statistically significant difference in interval between surgery and adjuvant chemotherapy between the groups.
Table 1
| Variable | Total (n=311) | Recurrence | P value | |
|---|---|---|---|---|
| Yes (n=241) | No (n=70) | |||
| Age, years, mean (SD) | 61.9 (9.4) | 61.5 (9.8) | 63.0 (7.9) | 0.257 |
| Male, n (%) | 195 (62.7) | 156 (64.7) | 39 (55.7) | 0.170 |
| BMI, kg/m2, mean (SD) | 23.2 (2.6) | 23.1 (2.6) | 23.5 (2.8) | 0.326 |
| Charlson age-comorbidity index ≥5, n (%) | 112 (36.0) | 87 (36.1) | 25 (35.7) | 0.953 |
| Preoperative CA 19-9, U/mL, median (IQR)* | 52.3 (15.2–202.0) | 69.7 (19.5–240.0) | 29.9 (4.3–84.1) | 0.018 |
| Tumor size on preoperative CT, cm, mean (SD) | 2.75 (1.34) | 2.89 (1.40) | 2.24 (0.95) | <0.001 |
| Radiologic splenic vessels invasion, n (%) | 174 (55.9) | 157 (65.1) | 17 (24.3) | <0.001 |
| Abutment/Encasement | 91 (52.3)/83 (47.7) | 84 (53.5)/73 (46.5) | 7 (41.2)/10 (58.8) | |
| SpA/SpV/Both | 28 (16.1)/55 (31.6)/91 (52.3) | 28 (17.8)/49 (31.2)/80 (51.0) | 0 (0.0)/6 (35.3)/11 (64.7) | |
| SUVmax on preoperative FDG-PET, mean (SD)† | 4.8 (2.5) | 4.9 (2.6) | 4.3 (2.3) | 0.126 |
| Combined adjacent organ resection, n (%) | 28 (9.0) | 26 (10.8) | 2 (2.9) | 0.041 |
| Differentiation, n (%)‡ | 0.393 | |||
| Well/Moderate | 267 (88.4) | 204 (87.6) | 63 (91.3) | |
| Poorly | 35 (11.6) | 29 (12.4) | 6 (8.7) | |
| T stage (AJCC 8th), n (%) | 0.002 | |||
| 1–2 | 236 (75.9) | 173 (71.8) | 63 (90.0) | |
| 3–4 | 75 (24.1) | 68 (28.2) | 7 (10.0) | |
| N stage (AJCC 8th), n (%) | <0.001 | |||
| 0 | 159 (51.1) | 108 (44.8) | 51 (72.9) | |
| 1–2 | 152 (48.9) | 133 (55.2) | 19 (27.1) | |
| Lymphovascular invasion, n (%) | 124 (39.9) | 107 (44.4) | 17 (24.3) | 0.002 |
| Perineural invasion, n (%) | 239 (76.8) | 198 (82.2) | 41 (58.6) | <0.001 |
| Pancreas resection margin positive, n (%) | 5 (1.6) | 0 (0.0) | 5 (7.1) | 0.224 |
| Radial resection margin positive, n (%) | 65 (20.9) | 62 (25.7) | 3 (4.3) | <0.001 |
| Pathologic splenic vessels invasion, n (%)§ | 74/221 (33.5) | 60/172 (34.9) | 14/49 (28.6) | 0.409 |
| Postoperative CA 19-9, U/mL, median (IQR)¶ | 18.8 (8.1–54.6) | 22.0 (9.3–66.9) | 11.7 (5.3–26.1) | 0.209 |
| Clinically relevant POPF, n (%) | 28 (9.0) | 20 (8.3) | 8 (11.4) | 0.421 |
| Postoperative complication, n (%) | 0.390 | |||
| Clavien Dindo classification I–II | 74 (23.8) | 62 (25.7) | 12 (17.1) | |
| Clavien Dindo classification III–V | 16 (5.1) | 11 (4.6) | 5 (7.1) | |
| Adjuvant chemotherapy, n (%) | 220 (70.7) | 172 (71.4) | 48 (68.6) | 0.651 |
| Adjuvant radiotherapy, n (%) | 52 (16.7) | 44 (18.3) | 8 (11.4) | 0.178 |
| Interval between surgery and adjuvant chemotherapy, days, median (IQR) | 36.0 (29.0–43.0) | 36.0 (30.3–44.8) | 34.5 (27.0–40.8) | 0.295 |
*, three hundred seven patients had preoperative CA 19-9 levels available for analysis. Excluded from 4 patients with missing preoperative values; †, two hundred fifty-seven patients had SUVmax value on preoperative FDG-PET available for analysis. Excluded from 54 patients with missing preoperative values; ‡, three hundred two patients had pathologic differentiation available for analysis. Excluded from 9 patients with missing pathologic data; §, two hundred twenty-one patients had pathologic splenic vessel invasion available for analysis. Excluded from 90 patients with missing pathologic data; ¶, three hundred eight patients had postoperative CA 19-9 levels available for analysis. Excluded from 3 patients with missing postoperative values. SD, standard deviation; BMI, body mass index; CA 19-9, carbohydrate antigen 19-9; IQR, interquartile range; CT, computed tomography; SpA, splenic artery; SpV, splenic vein; SUVmax, maximum standard uptake value; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; POPF, postoperative pancreatic fistula.
Definitions and comparisons between early and late recurrence
The potential early recurrence cutoff and associated PRS outcomes are shown in Table 2. The optimal length of RFS was 12 months (P=1.84×10−7) to distinguish between early recurrence (ER) and late recurrence (LR) based on the PRS.
Table 2
| Cutoff (months) | Potential early recurrence cohort | Potential late recurrence cohort | P value | |||||
|---|---|---|---|---|---|---|---|---|
| N | RFS (months) | PRS (months) | N | RFS (months) | PRS (months) | |||
| 3 | 21 | 1.9 | 8.6 | 220 | 9.4 | 12.1 | 5.15×10−3 | |
| 6 | 81 | 3.8 | 9.1 | 160 | 11.8 | 14.2 | 1.84×10−4 | |
| 9 | 129 | 5.0 | 9.1 | 112 | 15.0 | 16.6 | 3.20×10−7 | |
| 12 | 162 | 6.0 | 9.6 | 79 | 22.1 | 17.2 | 1.84×10−7 | |
| 15 | 185 | 6.8 | 10.5 | 56 | 29.1 | 18.5 | 3.00×10−5 | |
| 18 | 195 | 6.9 | 10.6 | 45 | 35.0 | 20.3 | 1.00×10−5 | |
Shown in italic is the optimal cutoff value. RFS, recurrence free survival; PRS, post recurrence survival.
Median RFS in patients with ER (<12 months, n=162) was 6.0 (95% CI: 5.1–6.9) months. Patients with recurrence after 12 months (LR, n=79) had a median RFS of 22.1 (95% CI: 17.7–26.5) months. The median OS in patients with ER (16.1 months, 95% CI: 14.7–17.5 months) was also significantly shorter than in those with LR (39.9 months, 95% CI: 33.2–46.6 months; P<0.001). The median PRS in patients with ER (9.6 months, 95% CI: 8.6–10.7 months) was significantly shorter than in those with LR (17.2 months, 95% CI: 15.2–19.2 months; P<0.001) (Figure 4).
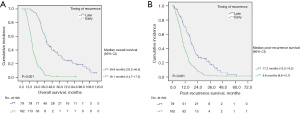
Patients’ demographics were not significantly differed between the ER and LR groups in terms of age, sex, BMI, or Charlson age-comorbidity index. Preoperative CA 19-9 (89.2 vs. 36.3 U/mL, P=0.016) and CEA (2.4 vs. 2.0 ng/mL, P=0.018) were significantly higher in the ER group than in the LR group. The tumor size on preoperative CT (3.09 vs. 2.49 cm, P=0.002) and SUVmax on preoperative FDG-PET (5.4 vs. 4.0, P<0.001) were significantly larger in the ER group than in the LR group. The presence of splenic vessel invasion (SVI) on preoperative CT was significantly more common in the ER group (78.4% vs. 38.0%, P<0.001). Combined adjacent organ resection was performed significantly more frequently in the ER group than in the LR group (13.6% vs. 5.1%, P=0.045). In terms of the pathologic results, lymphovascular invasion (50.6% vs. 31.6%, P=0.005) and a positive radial resection margin (29.6% vs. 17.7%, P=0.047) were significantly more frequent in the ER group than in the LR group. There were no statistically significant differences in postoperative CA 19-9, clinically relevant POPF, or postoperative complications between the groups. Adjuvant chemotherapy was performed more frequently in patients with LR (66.7% vs. 81.0%, P=0.021) (Table 3).
Table 3
| Variable | Timing of recurrence (months) | Site of recurrence | |||||
|---|---|---|---|---|---|---|---|
| Early (<12) (n=162) | Late (>12) (n=79) | P value | Isolated local (n=47) | Systemic (n=194) | P value | ||
| Age, years, mean (SD) | 61.8 (10.4) | 61.0 (8.2) | 0.569 | 61.3 (7.8) | 61.6 (10.2) | 0.858 | |
| Male, n (%) | 110 (67.9) | 46 (58.2) | 0.140 | 28 (59.6) | 128 (66.0) | 0.410 | |
| Charlson age-comorbidity index ≥5, n (%) | 63 (38.9) | 24 (30.4) | 0.197 | 12 (25.5) | 75 (38.7) | 0.093 | |
| BMI, kg/m2, mean (SD) | 23.0 (2.5) | 23.3 (2.7) | 0.445 | 23.2 (2.6) | 23.1 (2.6) | 0.734 | |
| Waiting time to surgery, month, mean (SD) | 0.9 (0.7) | 0.9 (0.5) | 0.498 | 0.9 (0.5) | 0.9 (0.7) | 0.309 | |
| Preoperative CA 19-9, U/mL, median (IQR)* | 89.2 (31.6–301.8) | 36.3 (13.5–108.5) | 0.016 | 66.7 (19.1–274.0) | 69.7 (19.3–230.0) | 0.579 | |
| Preoperative CEA, ng/mL, median (IQR) | 2.4 (1.4–4.7) | 2.0 (1.2–3.1) | 0.018 | 1.9 (1.2–3.5) | 2.5 (1.3–3.9) | 0.054 | |
| Neutrophil-to-lymphocyte ratio, mean (SD) | 2.1 (1.2) | 1.9 (1.1) | 0.140 | 2.1 (1.2) | 2.1 (1.1) | 0.990 | |
| Tumor size on preoperative CT, cm, mean (SD) | 3.09 (1.51) | 2.49 (1.05) | 0.002 | 2.87 (1.46) | 2.90 (1.39) | 0.901 | |
| Radiologic splenic vessel invasion, n (%) | 127 (78.4) | 30 (38.0) | <0.001 | 23 (48.9) | 134 (69.1) | 0.009 | |
| Abutment/Encasement | 71 (55.9)/56 (44.1) | 13 (43.3)/17 (56.7) | 11 (47.8)/12 (52.2) | 73 (54.5)/61 (45.5) | |||
| SpA/SpV/Both | 23 (18.1)/41 (32.3)/63 (49.6) | 5 (16.7)/8 (26.7)/17 (56.7) | 4 (17.4)/7 (30.4)/12 (52.2) | 24 (17.9)/42 (31.3)/68 (50.7) | |||
| SUVmax on preoperative FDG-PET, mean (SD)† | 5.4 (2.7) | 4.0 (2.1) | <0.001 | 4.7 (2.2) | 4.9 (2.7) | 0.575 | |
| Combined adjacent organ resection, n (%) | 22 (13.6) | 4 (5.1) | 0.045 | 6 (12.8) | 20 (10.3) | 0.626 | |
| Poorly differentiated, n (%)‡ | 20 (12.8) | 9 (11.7) | 0.805 | 3 (6.7) | 26 (13.8) | 0.191 | |
| T stage 3–4, n (%) | 51 (31.5) | 17 (21.5) | 0.107 | 12 (25.5) | 56 (28.9) | 0.649 | |
| N stage 1–2, n (%) | 94 (58.0) | 39 (49.4) | 0.205 | 24 (51.1) | 109 (56.2) | 0.526 | |
| Lymphovascular invasion, n (%) | 82 (50.6) | 25 (31.6) | 0.005 | 23 (48.9) | 84 (43.3) | 0.485 | |
| Perineural invasion, n (%) | 130 (80.2) | 68 (86.1) | 0.267 | 38 (80.9) | 160 (82.5) | 0.794 | |
| Pancreas resection margin positive, n (%) | 2 (1.2) | 3 (3.8) | 0.190 | 0 (0.0) | 5 (2.6) | 0.266 | |
| Radial resection margin positive, n (%) | 48 (29.6) | 14 (17.7) | 0.047 | 11 (23.4) | 51 (26.3) | 0.685 | |
| Pathologic Splenic vessel invasion, n (%)§ | 45/115 (39.1) | 15/57 (26.3) | 0.097 | 12/34 (35.3) | 48/138 (34.8) | 0.955 | |
| Postoperative CA 19-9, U/mL, median (IQR)¶ | 26.3 (10.3–94.2) | 12.7 (7.0–34.8) | 0.323 | 23.5 (9.5–54.5) | 20.5 (9.2–69.3) | 0.466 | |
| Clinically relevant POPF, n (%) | 17 (10.5) | 3 (3.8) | 0.077 | 4 (8.5) | 16 (8.2) | 0.953 | |
| Clavien Dindo classification ≥3, n (%) | 9 (5.6) | 2 (2.5) | 0.291 | 0 (0.0) | 11 (5.7) | 0.095 | |
| Adjuvant chemotherapy, n (%) | 108 (66.7) | 64 (81.0) | 0.021 | 37 (78.7) | 135 (69.6) | 0.214 | |
| Adjuvant radiotherapy, n (%) | 31 (19.1) | 13 (16.5) | 0.613 | 7 (14.9) | 37 (19.1) | 0.506 | |
| Interval between surgery and adjuvant chemotherapy, days, median (IQR) | 36.0 (30.0–45.0) | 36.0 (32.0–44.0) | 0.819 | 36.0 (29.0–44.0) | 36.0 (31.0–45.0) | 0.979 | |
*, two hundred thirty-seven patients had preoperative CA 19-9 levels available for analysis. Excluded from 4 patients with missing preoperative values; †, one hundred ninety-nine patients had SUVmax value on preoperative FDG-PET available for analysis. Excluded from 42 patients with missing preoperative values; ‡, two hundred thirty-three patients had pathologic differentiation available for analysis. Excluded from 8 patients with missing pathologic data; §, one hundred seventy-two patients had pathologic splenic vessel invasion available for analysis. Excluded from 69 patients with missing pathologic data; ¶, two hundred thirty-eight patients had postoperative CA 19-9 levels available for analysis. Excluded from 3 patients with missing postoperative values. SD, standard deviation; BMI, body mass index; CA 19-9, carbohydrate antigen 19-9; IQR, interquartile range; CEA, carcinoembryonic antigen; CT, computed tomography; SpA, splenic artery; SpV, splenic vein; SUVmax, maximum standard uptake value; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; POPF, postoperative pancreatic fistula.
Comparison between isolated local and systemic recurrence
We found 47 patients (19.5%) recurred at only local sites and 194 patients (80.5%) experienced systemic recurrence (distant ± local). The patients most often experienced liver only (n=48, 19.9%), followed by isolated local (n=47, 19.5%), local with distant (n=45, 18.7%), peritoneal seeding (n=33, 13.7%), lung only (n=18, 7.5%), distant LNs (n=16, 6.6%), or other sites (n=5, 2.1%) recurrence (Table 4).
Table 4
| Site of first recurrence | Patients with recurrence (n=241) |
|---|---|
| Isolated local | 47 (19.5%) |
| Systemic | 194 (80.5%) |
| Local + distant | 45 (18.7%) |
| Distant | 149 (61.8%) |
| Liver only | 48 (19.9%) |
| Lung only | 18 (7.5%) |
| Multiple distant site | 29 (12.0%) |
| Distant lymph node | 16 (6.6%) |
| Peritoneal seeding | 33 (13.7%) |
| Others | 5 (2.1%) |
The median OS was significantly longer for patients with isolated local recurrence (29.1 months, 95% CI: 22.0–36.2 months) than patients with systemic recurrence (19.6 months, 95% CI: 16.7–22.5 months; P=0.007). The median PRS in patients with isolated local recurrence (15.3 months, 95% CI: 9.8–20.8 months) was significantly longer than in those with systemic recurrence (11.0 months, 95% CI: 9.3–12.7 months; P=0.024). Median RFS in patients with an isolated local recurrence (10.8 months, 95% CI: 8.2–13.4 months) was also significantly longer than in those with systemic recurrence (7.7 months, 95% CI: 6.8–8.6 months; P=0.029) (Figure 5).

Radiologic SVs invasion was more common in patients with systemic recurrence than with isolated local recurrence (48.9% vs. 69.1%, P=0.009). Other preoperative factors were not significantly different between the two groups. The pathologic results and postoperative outcomes were also similar between the groups (Table 3).
Predictors for early systemic recurrence
The results of univariate and multivariate Cox proportional hazard regression analysis are presented in Table 5. In univariate analysis, preoperative CA 19-9 ≥500 U/mL, preoperative CEA ≥5 ng/dL, NLR ≥2.0, tumor size on preoperative CT ≥3.0 cm, SVs invasion on preoperative CT, SUVmax on preoperative FDG-PET ≥5.0, open surgery, combined adjacent organ resection, T3–4 stage, N1–2 stage, lymphovascular invasion, positive radial resection margin, postoperative CA 19-9 ≥37 U/mL, and no adjuvant chemotherapy were statistically significant predictors for early systemic recurrence. In a multivariate Cox regression model that included the significant factors identified in the univariate analyses, preoperative CA 19-9 ≥500 U/mL [odds ratio (OR) 2.037, P=0.035], radiologic SVs invasion (OR 5.014, P<0.001), positive radial resection margin (OR 2.638, P<0.001), and no adjuvant chemotherapy (OR 2.084, P=0.001) were significant predictors of an early systemic recurrence.
Table 5
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age (≥70 years) | 1.425 (0.970–2.093) | 0.071 | |||
| Gender (male) | 1.303 (0.910–1.865) | 0.149 | |||
| Charlson age-comorbidity index (≥5 points) | 1.191 (0.845–1.680) | 0.319 | |||
| BMI (<20 kg/m2) | 0.817 (0.470–1.421) | 0.474 | |||
| Preoperative CA 19-9 (≥500 U/mL) | 2.164 (1.378–3.398) | 0.001 | 2.037 (1.053–3.943) | 0.035 | |
| Preoperative CEA (≥5 ng/dL) | 1.644 (1.081–2.502) | 0.020 | 0.982 (0.589–1.635) | 0.943 | |
| Neutrophil-to-lymphocyte ratio (≥2.0) | 1.471 (1.045–2.071) | 0.027 | 1.066 (0.705–1.612) | 0.760 | |
| Tumor size on preoperative CT (≥3.0 cm) | 1.602 (1.145–2.242) | 0.006 | 0.795 (0.495–1.275) | 0.341 | |
| Splenic vessels invasion on preoperative CT (+) | 4.600 (3.029–6.985) | <0.001 | 5.014 (2.811–8.943) | <0.001 | |
| SUVmax on FDG-PET (≥5.0) | 1.638 (1.127–2.382) | 0.010 | 1.129 (0.740–1.722) | 0.573 | |
| Method of surgery (open surgery) | 1.657 (1.171–2.343) | 0.004 | 1.253 (0.834–1.882) | 0.278 | |
| Combined adjacent organ resection (+) | 2.038 (1.239–3.351) | 0.005 | 1.184 (0.631–2.220) | 0.599 | |
| T stage (3–4) | 1.588 (1.104–2.285) | 0.013 | 1.067 (0.649–1.754) | 0.799 | |
| N stage (1–2) | 1.581 (1.127–2.217) | 0.008 | 0.865 (0.557–1.343) | 0.518 | |
| Differentiation (poorly) | 1.500 (0.912–2.466) | 0.110 | |||
| Lymphovascular invasion (+) | 1.691 (1.208–2.367) | 0.002 | 1.251 (0.837–1.870) | 0.275 | |
| Perineural invasion (+) | 1.403 (0.915–2.150) | 0.120 | |||
| Pancreas resection margin (+) | 0.903 (0.223–3.647) | 0.886 | |||
| Radial resection margin (+) | 2.370 (1.641–3.422) | <0.001 | 2.638 (1.691–4.114) | <0.001 | |
| Pathologic splenic vessel invasion (+) | 1.357 (0.900–2.048) | 0.145 | |||
| Postoperative CA 19-9 (≥37 U/mL) | 2.093 (1.483–2.955) | <0.001 | 1.236 (0.745–2.052) | 0.412 | |
| Clinically relevant POPF (+) | 1.240 (0.713–2.156) | 0.445 | |||
| Clavien Dindo classification (grade ≥3) | 1.494 (0.760–2.938) | 0.244 | |||
| Adjuvant Chemotherapy (−) | 1.622 (1.143–2.301) | 0.007 | 2.084 (1.367–3.177) | 0.001 | |
| Adjuvant Radiotherapy (−) | 0.801 (0.526–1.220) | 0.301 | |||
CI, confidence interval; BMI, body mass index; CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CT, computed tomography; SUVmax, maximum standard uptake value; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; POPF, postoperative pancreatic fistula.
Discussion
Several previous studies reported that clinicopathological factors such as tumor size, LN metastasis, surgical resection margin status, tumor markers (CA 19-9), SUVmax on FDG-PET, and systemic inflammatory markers (neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, and Glasgow prognostic score) were associated with the prognosis following pancreatectomy for potentially R-PDAC (20-24). However, depending on the anatomical location of the PDAC, the prognosis and survival may vary (9,25). Studies focusing on left-sided PDAC with resectable status are rare. The present study is meaningful as it analyzed postoperative factors as well as preoperative factors that affect the prognosis in only resectable left-sided PDAC.
Due to the poor prognosis of PDAC with its high rate of recurrence following curative surgery, studies on the definition and risk factors of ER have been recently published (26-28). Groot et al. (27) reported that a RFS of 12 months is the optimal threshold for differentiating between early and late recurrence, based on the subsequent prognosis according to the minimum P value approach. In the present study, 12 months was also found to be the optimal length of RFS to distinguish between ER and LR for resectable left-sided PDAC. Studies on the prognosis according to the pattern of the first recurrence site following pancreatectomy are also being reported. Recently, two studies reported that hepatic relapse was associated with a poor prognosis (29,30). However, both studies excluded left-sided PDAC after distal pancreatectomy, and studies analyzing the survival outcomes according to the recurrence pattern of left-sided PDAC are rare. In the present study, we compared survival outcomes according to the patterns of the first recurrence site (isolated local versus systemic). It was confirmed that systemic recurrence was associated with poor survival outcomes relative to those with an isolated local recurrence.
In patients with PDAC, preoperative radiologic findings play an important role in determining resectability and treatment plans, and in predicting the outcome. In particular, since the anatomical location of the pancreas is close to the major vessels such as the celiac artery (CA), SMA, common hepatic artery (CHA), portal vein (PV), and superior mesenteric vein (SMV), the relationship between the tumor and the major vessels is important to decide on resectability. According to the NCCN guidelines, SVs invasion in left-sided PDAC is classified as R-PDAC, unlike PV-SMV and CA-SMA invasion. Although recent studies reported that pathological infiltration of SV is associated with a worse prognosis (31-33), resectable left-sided PDAC with SVs invasion alone is considered “anatomically” resectable disease. However, recent studies reported that radiologic SVs invasion is associated with a poor prognosis, suggesting a need for NAT (34-36). The present study also showed a poor prognostic impact of radiologic SVs invasion in anatomically resectable left-sided PDAC. In contrast, a recently published multicenter study reported that on preoperative imaging, patients with tumors interfacing with the SV did not have a worse survival, although they achieved fewer R0 resections (37). The authors claimed that vascular invasion is not in itself a marker of worse tumor biology, but rather, it reflects the likelihood of an incomplete resection, which is correlated with worse survival outcomes. However, we confirmed that radiologic SVs invasion is a predictor of poor survival regardless of a positive resection margin in multivariate analysis.
The mechanism by which radiologic SVs invasion leads to a poor outcome of left-sided PDAC has not been identified. The radiologic and pathologic SVs invasion findings are not necessarily consistent. The sensitivity for a correlation between radiologic and pathologic SVs invasion has been reported to range from 33–37% (32,33,35). Kitamura et al. (38) suggested that radiologic and pathologic splenic artery involvement are not consistent because the periarterial plexus us thick, making it difficult for tumors to invade the arterial wall pathologically. According to our study results, although not pathologic SVs invasion, radiologic SVs invasion is a significant predictor of early systemic recurrence. Therefore, radiologic SVs invasion does not only indicate pathologic SVs invasion, but includes other factors affecting prognosis. For example, in present study, a higher perineural invasion was confirmed in patients with radiologic SVs invasion but no pathologic SVs invasion (53/64, 82.8%) than in the entire study cohorts (239/311, 76.8%). In addition, while there are many studies on the prognostic impact of the depth of PV-SMV invasion in PDAC in the head of the pancreas (39,40), studies on the prognostic impact of the depth of SVs invasion in left-sided PDAC are lacking. Therefore, additional studies on the correlation between radiologic and pathologic SVs invasion with tumor biology in left-sided PDAC are needed.
The International Association of Pancreatology (IAP) defined patients with borderline resectable PDAC according to three distinct dimensions: anatomical, biological, and conditional (41). Biological factors include clinical findings suspicious for (but unproven) distant metastases or regional LN metastases diagnosed by biopsy or FDG-PET, and serum CA 19-9 ≥500 U/mL. According to a large cohort study of patients with potentially R-PDAC, in patients with preoperative CA 19-9 levels greater than 500 U/mL, the resectability ratio was less than 70% and the median survival time after pancreatectomy was less than 20 months (42). It was on this basis that the IAP consensus view was that a preoperative CA 19-9 of 500 U/mL should be included in the definition of borderline resectable PDAC as a biological factor. Similarly, our study showed that the patients with preoperative CA 19-9 ≥500 U/mL were associated with a poor prognosis following upfront surgery. Radiologic SVs invasion was also confirmed to be a preoperative predictor of early systemic recurrence. Therefore, while a left-sided PDAC patient with radiologic SVs invasion has an anatomically resectable status, they may be considered to have a biologically borderline status due to preoperative CA 19-9 elevation (≥500 U/mL). Since radiologic SVs invasion and preoperative CA 19-9 elevation (≥500 U/mL) are not only risk factors for ER but also risk factors for systemic recurrence, NAT, which is a systemic treatment, may be considered before upfront surgery to improve survival outcomes. Therefore, future clinical trials on NAT targeting resectable left-sided PDAC with radiologic SVs invasion or preoperative CA 19-9 elevation (≥500 U/mL) should be conducted to confirm these hypotheses.
The present study has several limitations. First, this was a single-institutional, retrospective study, so our results may have limited generalizability. A multi-institutional, prospective, randomized, controlled trial with data on the neoadjuvant approach is required to confirm the prognostic impact of radiologic SVs invasion in resectable left-sided PDAC. Second, pathological re-evaluation of the surgical specimen was not performed and the pathologic results were only obtained from medical records in the current study. There were many missing values for the presence of pathologic SVs invasion and accordingly, our study could not identify the prognostic impact of pathological SVs invasion in resectable left-sided PDAC. Third, we did not analyze the splenic artery and splenic vein separately since the study cohort would have been heterogeneously divided into small groups. Additional investigations involving a larger sample size with homogenous characteristics and therapeutic approaches are required to clarify the significance of splenic artery and vein invasion, respectively.
In conclusion, radiologic SVs invasion may be considered a biologically borderline status in patients with anatomically resectable left-sided PDAC. Assessing radiological SVs invasion can be useful in predicting patient outcomes and determining the optimal therapeutic approach in these patients. Future clinical trials on NAT targeting these patients should be conducted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-304/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-304/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-304/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-304/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Asan Medical Center (Approval No. 2019–1404) and informed consent was waived due to the retrospective study design. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med 1992;326:455-65. [Crossref] [PubMed]
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Youngwirth LM, Nussbaum DP, Thomas S, et al. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18 243 patients. J Surg Oncol 2017;116:127-32. [Crossref] [PubMed]
- Birrer DL, Golcher H, Casadei R, et al. Neoadjuvant Therapy for Resectable Pancreatic Cancer: A New Standard of Care. Pooled Data From 3 Randomized Controlled Trials. Ann Surg 2021;274:713-20. [Crossref] [PubMed]
- Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371-6. [Crossref] [PubMed]
- Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg 1996;223:506-11; discussion 511-2. [Crossref] [PubMed]
- Lof S, Korrel M, van Hilst J, et al. Impact of Neoadjuvant Therapy in Resected Pancreatic Ductal Adenocarcinoma of the Pancreatic Body or Tail on Surgical and Oncological Outcome: A Propensity-Score Matched Multicenter Study. Ann Surg Oncol 2020;27:1986-96. [Crossref] [PubMed]
- Nelson DW, Chang SC, Grunkemeier G, et al. Resectable Distal Pancreas Cancer: Time to Reconsider the Role of Upfront Surgery. Ann Surg Oncol 2018;25:4012-9. [Crossref] [PubMed]
- Nassour I, Adam MA, Kowalsky S, et al. Neoadjuvant therapy versus upfront surgery for early-stage left-sided pancreatic adenocarcinoma: A propensity-matched analysis from a national cohort of distal pancreatectomies. J Surg Oncol 2021;123:245-51. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery 2003;133:521-7. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8 ed. Springer International Publishing; 2017.
- Strobel O, Hank T, Hinz U, et al. Pancreatic Cancer Surgery: The New R-status Counts. Ann Surg 2017;265:565-73. [Crossref] [PubMed]
- Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol 2010;171:624-32. [Crossref] [PubMed]
- Yamamoto T, Sugiura T, Mizuno T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2015;22:677-84. [Crossref] [PubMed]
- Matsumoto I, Murakami Y, Shinzeki M, et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: A multi-center retrospective study. Pancreatology 2015;15:674-80. [Crossref] [PubMed]
- Åkerberg D, Ansari D, Andersson R. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. World J Gastroenterol 2016;22:6424-33. [Crossref] [PubMed]
- Sierzega M, Lenart M, Rutkowska M, et al. Preoperative Neutrophil-Lymphocyte and Lymphocyte-Monocyte Ratios Reflect Immune Cell Population Rearrangement in Resectable Pancreatic Cancer. Ann Surg Oncol 2017;24:808-15. [Crossref] [PubMed]
- La Torre M, Nigri G, Cavallini M, et al. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol 2012;19:2917-23. [Crossref] [PubMed]
- Sung MK, Park Y, Kwak BJ, et al. Comparison of Characteristics and Survival Rates of Resectable Pancreatic Ductal Adenocarcinoma according to Tumor Location. Biomedicines 2021;9:1706. [Crossref] [PubMed]
- Guo SW, Shen J, Gao JH, et al. A preoperative risk model for early recurrence after radical resection may facilitate initial treatment decisions concerning the use of neoadjuvant therapy for patients with pancreatic ductal adenocarcinoma. Surgery 2020;168:1003-14. [Crossref] [PubMed]
- Groot VP, Gemenetzis G, Blair AB, et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg 2019;269:1154-62. [Crossref] [PubMed]
- Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of pancreatic cancer after pancreatectomy based on the difference in the prognosis. Pancreatology 2014;14:524-9. [Crossref] [PubMed]
- Suenaga M, Fujii T, Kanda M, et al. Pattern of first recurrent lesions in pancreatic cancer: hepatic relapse is associated with dismal prognosis and portal vein invasion. Hepatogastroenterology 2014;61:1756-61. [PubMed]
- Groot VP, Rezaee N, Wu W, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg 2018;267:936-45. [Crossref] [PubMed]
- Kanda M, Fujii T, Sahin TT, et al. Invasion of the splenic artery is a crucial prognostic factor in carcinoma of the body and tail of the pancreas. Ann Surg 2010;251:483-7. [Crossref] [PubMed]
- Partelli S, Crippa S, Barugola G, et al. Splenic artery invasion in pancreatic adenocarcinoma of the body and tail: a novel prognostic parameter for patient selection. Ann Surg Oncol 2011;18:3608-14. [Crossref] [PubMed]
- Mizumoto T, Toyama H, Asari S, et al. Pathological and Radiological Splenic Vein Involvement are Predictors of Poor Prognosis and Early Liver Metastasis After Surgery in Patients with Pancreatic Adenocarcinoma of the Body and Tail. Ann Surg Oncol 2018;25:638-46. [Crossref] [PubMed]
- Kang JS, Choi YJ, Byun Y, et al. Radiological tumour invasion of splenic artery or vein in patients with pancreatic body or tail adenocarcinoma and effect on recurrence and survival. Br J Surg 2021;109:105-13. [Crossref] [PubMed]
- Kawai M, Hirono S, Okada KI, et al. Radiographic Splenic Artery Involvement Is a Poor Prognostic Factor in Upfront Surgery for Patients with Resectable Pancreatic Body and Tail Cancer. Ann Surg Oncol 2021;28:1521-32. [Crossref] [PubMed]
- Hyun JJ, Rose JB, Alseidi AA, et al. Significance of radiographic splenic vessel involvement in the pancreatic ductal adenocarcinoma of the body and tail of the gland. J Surg Oncol 2019;120:262-9. [Crossref] [PubMed]
- Alemi F, Jutric Z, Marshall GR, et al. Preoperative imaging characteristics predict poor survival and inadequate resection for left-sided pancreatic adenocarcinoma: a multi-institutional analysis. HPB (Oxford) 2020;22:1216-21. [Crossref] [PubMed]
- Kitamura K, Esaki M, Sone M, et al. Prognostic Impact of Radiological Splenic Artery Involvement in Pancreatic Ductal Adenocarcinoma of the Body and Tail. Ann Surg Oncol 2022;29:7047-58. [Crossref] [PubMed]
- Nakao A, Kanzaki A, Fujii T, et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg 2012;255:103-8. [Crossref] [PubMed]
- Ratnayake CBB, Shah N, Loveday B, et al. The Impact of the Depth of Venous Invasion on Survival Following Pancreatoduodenectomy for Pancreatic Cancer: a Meta-analysis of Available Evidence. J Gastrointest Cancer 2020;51:379-86. [Crossref] [PubMed]
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- Hartwig W, Strobel O, Hinz U, et al. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol 2013;20:2188-96. [Crossref] [PubMed]



