Management of anaplastic thyroid cancer
Introduction
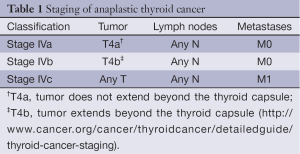
Anaplastic thyroid cancer (ATC) occurs in less than 2% of all thyroid cancer cases, affecting 1 to 2 individuals per million every year in the United States, and it is almost uniformly lethal. Patients are usually in their 6th or 7th decade of life at presentation, have an average median survival of 5 months, and less than 20% are alive 1 year after diagnosis (1,2). Due to the extremely aggressive behavior of ATC, the American Joint Committee on Cancer (AJCC) defines all of its stages as stage IV. Depending on the extension of the primary tumor, lymph node involvement, or presence of distant metastases (DM), ATC staging is divided into stage IVa, IVb, and IVc (Table 1). Although survival rates have not significantly improved in six decades, multimodality treatment, including surgery, radiation, chemotherapy, and targeted therapy, is considered the best strategy for improving outcome in patients diagnosed with ATC (3).
Full table
Pathogenesis
Different histopathologic growth patterns of ATC have been described, including spindle, pleomorphic, and squamoid morphologies. One of these patterns may predominate in a given tumor, or the tumor may show a mixed feature of two, or even all three, types. All three growth patterns have common distinctive features of dedifferentiated behavior, such as giant cells, numerous mitotic figures and atypical mitoses, extensive necrosis surrounded by inflammatory infiltrates and occasionally osteoclast-like giant cells, as well as, less commonly, the presence of neoplastic bone and cartilage (4-7). Although useful for diagnosis of ATC on histo- and cytopathology, histopathologic growth patterns do not appear to be associated with patient prognosis (8,9).
ATC is thought to originate in differentiated thyroid cancers of follicular cell origin, as a result of dedifferentiation. Up to 80% of ATC occurs in the setting of a long-standing goiter, possibly in the background of an undiagnosed, well-differentiated thyroid cancer (8). Dedifferentiation is associated with gains and deletions in multiple chromosomal regions and involves a complex process involving multiple events, including cell cycle derangement and signal transduction pathway disturbances (10-12).
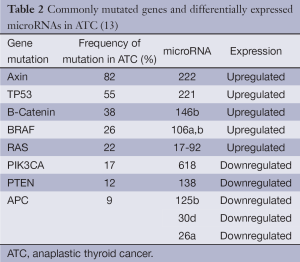
Several mutations have been described in ATCs (Table 2). Some, such as those in the BRAF and RAS oncogenes, are also commonly found in differentiated thyroid cancers, implying that these mutations may be early events in cancer formation (14-17). PIK3CA and PTEN gene mutations also occur in both differentiated thyroid cancer and ATC (14,16,17). Mutant PIK3CA and aberrant activation of the PI3K/Akt pathway were found in greater than 50% of ATCs. These abnormalities are believed to play an important role in thyroid cancer progression, as they have been suggested to promote progression of adenomas to follicular thyroid cancer and ATC based on a frequency of mutation/activation of this pathway that is relatively higher in cancer than in benign tumors (18,19).
The TP53 tumor suppressor gene mutation, on the other hand, is found almost exclusively in ATCs and likely represents a late event in dedifferentiation (20,21). This hypothesis is supported by experiments achieving redifferentiation of ATC tissue and restoration of cellular response to physiologic stimuli after re-expression of wild-type p53 (22). Decreased E-Cadherin and B-Catenin expression has also been found in poorly differentiated thyroid cancer and ATC. Loss of both adhesion molecules is associated with progressive loss of tumor differentiation and epithelial-to-mesenchymal transition, and low membrane B-Catenin expression and CTNNB1 exon3 mutation have been associated with poor prognosis in ATC (18,23,24). Additional dysregulated genetic events, such as microRNA and epigenetic alterations, may also contribute to ATC pathogenesis (2,25,26) (Table 2). Currently, most clinical management guidelines, such as those of the American Thyroid Association and the European Thyroid Association, do not recommend the use of molecular studies for diagnosis or management of ATC, as there is insufficient evidence of their clinical utility (9).
Clinical presentation and diagnosis
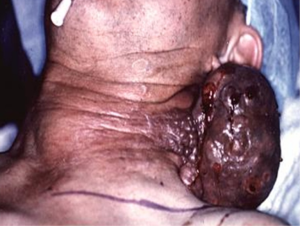
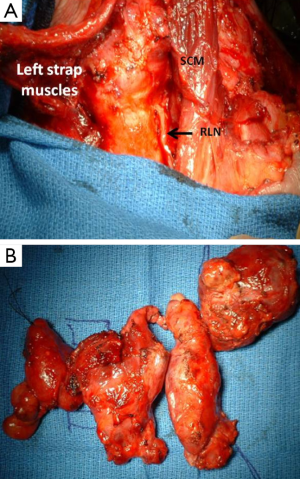
Most patients with ATC present with a rapidly enlarging neck mass (Figure 1) and locoregional symptoms, such as dyspnea, dysphagia, and neck pain. Other symptoms of ATC can be related to invasion in any neck structure, including the recurrent laryngeal nerve (RLN) (causing hoarseness), parasympathetic chain (causing Horner’s syndrome), or even carotid arteries (causing stroke, hematoma).
Approximately 40% of patients with ATC initially present with cervical lymphadenopathy, and up to 43% of patients have DM, most commonly to the lung, followed by bone and brain metastases (1).
The most important management consideration in patients with ATC is a rapid and accurate assessment of the disease burden, since tumor doubling time can be very short (i.e., days), therefore acutely compromising the airway in these patients and in some cases, rendering the tumor unresectable. Airway assessment and management should always have the highest priority and include a direct laryngoscopy and bronchoscopy if tracheal invasion is suspected.
As with any thyroid mass, a fine needle aspiration (FNA) should be performed first. This often secures the diagnosis (27). If the FNA is nondiagnostic, a core needle or open biopsy should be undertaken (9). The differential diagnosis for ATC includes poorly differentiated thyroid cancer, large cell lymphomas, medullary thyroid carcinoma, direct extension of a laryngeal carcinoma, primary squamous cell carcinoma of the thyroid, and metastatic melanoma (6,9).
Laboratory evaluation in patients with ATC should include complete blood count, basic metabolic profile, liver function, coagulation factors, and thyroid function tests (9). Thyrotoxicosis, hypocalcemia, and leukocytosis have all been described in patients with ATC. Additionally, since ATC occurs most commonly in patients who are elderly and some of these patients suffer from dysphagia and weight loss, a thorough nutritional assessment, including measuring albumin and/or pre-albumin levels, should be performed preoperatively.
Cross-sectional imaging such as CT and MRI of the neck and chest should be obtained prior to surgery to assess the extent of the tumor and degree of invasion of adjacent structures (Figure 2). Such information is critical for operative planning and/or to determine whether neoadjuvant therapy is indicated. Radiologic studies should not delay urgent therapeutic intervention and should be scheduled expeditiously. High-resolution ultrasound is a convenient, rapid, and easy imaging test for assessing tumor extension, involvement of central or lateral nodes, and invasion into adjacent structures (9). In patients with symptoms suggestive of tumor involvement, esophagogastroduodenoscopy (EGD) and/or bronchoscopy can assess esophageal or tracheal involvement, respectively.
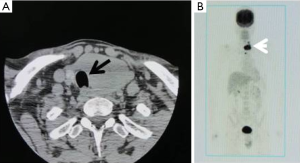
Although, as with any other cancer, a complete staging should be performed before treatment, this process should not delay primary management of ATC (e.g., biopsy to diagnose DM). CT imaging of the head, chest, abdomen, and pelvis are helpful in ruling out DM. 18FDG PET CT has recently gained favor for ATC staging since it appears to be more accurate at detecting DM than routine total body CT (28). Additionally, high FDG uptake values on PET have been correlated with poor survival in ATC (28). Additional tests, such as serum markers, should be used if another primary tumor is suspected. Finally, a biopsy of metastases, with or without immunohistochemistry, can confirm the diagnosis of metastatic ATC (9).
Surgical treatment
There are two cardinal rules for the management and surgical planning of patients with ATC: (I) assessment of airway; and (II) expeditious treatment (“Time is of the essence”).
Maintaining and securing a patent airway in patients with ATC can be challenging. Routine tracheostomy is not recommended, does not improve quality of life or prolong life, and is best avoided unless there is impending airway compromise (29). Recommendations for tracheostomy include: (I) acute airway distress; (II) unresectable tumors that would not benefit from debulking; or (III) mild dyspnea unresponsive to corticosteroids. A tracheostomy should be done in the operating room and by an experienced surgeon since large tumor mass and bleeding can obscure visibility. Tracheal stents can be useful for airway stabilization in the mid-trachea, but are rarely helpful in the subglottic area. The option of palliation without tracheostomy should be given to patients with unresectable ATC because tumor plugging, erosion, and bleeding can result from tracheostomy placement in these patients, significantly impairing their quality of life.
Surgical intervention should be determined based on preoperative staging. A good rule of thumb is that all patients with stage IVa or stage IVb, in which grossly negative margins (R1) can be obtained, should have a resection since complete resection is associated with prolonged disease-free and overall survival (1,30,31) (Figure 3). In the 2-15% of patients with ATC who present with stage IVa (intrathyroidal tumor), a total thyroidectomy with a therapeutic central and lateral neck node dissection is recommended. This recommendation also applies to stage IVb tumors, although neoadjuvant preoperative radiotherapy (XRT) can sometimes be considered in order to downstage locally unresectable disease and subsequently enable complete gross resection. However, here again, airway preservation should always have the highest priority.
Gross resection, and not debulking, should be the main goal in patients with ATC, but the extent of resection should be carefully weighed against the potentially devastating morbidity of certain procedures. Planned limited resection of the trachea or larynx may be performed with minimal morbidity; however, laryngectomy or esophagectomy are associated with high morbidity rates and are usually not undertaken in the setting of such an aggressive cancer with short survival times. It is also not reasonable to perform extended resections if a gross tumor is anticipated to be left behind in the neck or superior mediastinum.
In the special, and very rare, circumstance in which a microscopic focus of ATC is found incidentally within a differentiated thyroid cancer after thyroidectomy, the appropriate extent of thyroidectomy is unclear as there is no outcome data to support a specific surgical strategy. Although certain centers advocate a more conservative approach when an incidental focus of ATC is found, many advocate performing a completion thyroidectomy (32). In such clinical scenarios, it is reasonable to adapt an approach that is best for the differentiated thyroid cancer (i.e., the non-ATC component of the malignancy). The benefit of adjuvant radiotherapy with or without chemotherapy has not clearly been proven in this setting, and the ATA guidelines recommend close observation with frequent anatomic imaging (9).
Palliative resection of the primary tumor in stage IVc patients should be considered if possible, to avoid future, or treat current, airway compromise or esophageal obstruction; such an approach has the potential to prolong survival and enhance quality of life (33).
Complications of ATC resections include hemorrhage, chylous fistulae, vocal cord paralysis, surgical site infection, dysphagia, salivary fistulae, and hypoparathyroidism.
Systemic and external beam radiation (XRT) treatment
In patients with ATC, the benefit of systemic chemotherapy and XRT is unclear but may be considered in three clinical settings: (I) as neoadjuvant therapy for locoregional ATC to downstage the tumor; (II) as adjuvant therapy after complete ATC resection or for low-volume locoregional and distant residual disease; and (III) as palliative therapy. There is no standard chemotherapy regimen for ATC, and, in the setting of unresectable or symptomatic disease, systemic chemotherapy is best employed in a clinical trial, as no agent(s) has provided a survival advantage significant enough to warrant its use outside of a clinical trial.
Adjuvant therapy in the form of XRT or chemotherapy should be started as soon as the patient recovers from surgery, usually within 2 or 3 weeks after surgery (9). Some, but not all, retrospective studies suggest that multimodal therapy with a combination of surgery (R0 or R1) and definitive XRT (with or without concurrent chemotherapy) achieves better survival rates (1,9). Even patients who have R2 resections or unresected disease and have good performance status should be offered radiation because this may result in better local disease control.
In patients with nonmetastatic ATC and good performance status, cytotoxic chemotherapy should be added to XRT. In the past, doxorubicin has been used most often; however, more recent drugs, such as cisplatin or paclitaxel, have been used as radiosensitizing agents, and studies of these drugs have reported better 1-year survival rates, as compared to historical controls (34-37).
In cases of advanced metastatic disease (stage IVc) related to ATC, no cytotoxic or targeted systemic therapy has definitively been shown to have curative potential or to prolong survival rates. Therefore, the patient’s disease status, performance status, and his or her wishes must be considered before choosing the best approach, as most therapies have risks and side effects (dysphagia, odynophagia, and chemotherapy-induced neutropenia). Determining whether the patient has symptomatic or life-threatening focal disease (calling for treatment with “palliative” XRT) or more diffuse systemic disease progression (calling for systemic therapy) helps in determining the appropriate therapeutic approach. Doxorubicin is the only FDA-approved drug for systemic therapy that may be used to treat ATC, and, while it has achieved modest effects against advanced ATC, it is often used in combination with other modalities (38).
A US national cancer registry study shows a longer median survival rate with combined therapies: for stage IVa ATC, the median survival is 11.2 months using a combination of all three modalities (surgery, XRT, and chemotherapy) vs. 9.3 months if surgery and radiation were performed without chemotherapy. For stage IVc ATC, the median survival is 4.9 vs. 3.5 months, respectively (39).
As no systemic therapy has been shown to improve the survival rate or quality of life in patients with advanced ATC, new clinical trials with targeted therapies are needed, and patients should be enrolled in them as soon as possible. In a randomized study, fosbretabulin, a vascular disrupting agent, showed some benefits when added to paclitaxel and carboplatin (40). A recent phase II trial in 20 patients with advanced disease who were treated with daily sorafenib, a tyrosine kinase inhibitor, showed overall median progression-free survival of 1.9 months, with a median and a 1-year survival rate of 3.9 months and 20%, respectively (41). In one patient with a BRAF V600E-mutated ATC, the use of vemurafenib resulted in nearly complete tumor regression (42). Further, whole-exome sequencing in pretreated, responsive and then resistant tumor tissue to everolimus showed mTOR pathway activation in ATC as a target for therapy in clinical trials (43).
In the case of advanced ATC with DM, there is no standard systemic therapy recommended. Metastases are common to the lung and liver, which, in most cases, present with numerous lesions. In such cases, depending on patient functional status and tolerance, treatment with focal radiotherapy or radiofrequency ablation may be considered for palliation.
Surveillance and follow up
Patients with ATC who have had a complete resection without persistent disease should undergo aggressive surveillance with cross-sectional imaging every 1-3 months for the first year, and every 4-6 months thereafter. FDG PET should be considered as a useful tool to monitor recurrence or to assess the success of treatment with adjuvant therapies. Thyroglobulin measurements and radioactive iodine scanning are not useful in ATC (9).
Prognosis
Several studies have examined the factors affecting prognosis in patients with ATC (Table 3). These prognostic factors, including patient age, tumor size, and clinical stage, should be considered when evaluating patients for treatment (9). A vast majority of patients will ultimately die from their disease and a thorough discussion regarding prognosis should be held with patients, so they understand the impact of their disease on their quality of life, as well as the potential benefit of participating in experimental clinical trials. Additionally, empathy to comfort and pain issues in the final moments of a patient’s life are of utmost importance. A discussion regarding “do not resuscitate” (DNR) or “allow natural death” (AND) orders should be implemented by the treating clinician. An AND order may have the advantage of less ambiguity in emergency situation requiring potential life extending measures such as intubation.
Full table
Conclusions
ATC is a deadly disease with dismal long-term survival rates. Treatment strategy should be based on recommendations from a multidisciplinary team including surgeons, medical oncologists, endocrinologists and radiation oncologists. Best management practices include rapid assessment of disease burden, including potential airway compromise, adequate staging, and operative therapy with the goal of gross complete resection combined with chemo- and/or radiotherapy. Although there have been advances in understanding the molecular pathogenesis of this aggressive cancer over the last two decades, more work needs to be done to identify suitable targets for successful tumor-directed therapy.
Acknowledgements
We would like to thank Nancy Parrish for helping with editing and formatting of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Kebebew E, Greenspan FS, Clark OH, et al. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005;103:1330-5. [PubMed]
- Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 2010;22:486-97. [PubMed]
- Kebebew E. Anaplastic thyroid cancer: rare, fatal, and neglected. Surgery 2012;152:1088-9. [PubMed]
- Bronner MP. LiVolsi VA. Spindle cell squamous carcinoma of the thyroid: an unusual anaplastic tumor associated with tall cell papillary cancer. Mod Pathol 1991;4:637-43. [PubMed]
- Gaffey MJ, Lack EE, Christ ML, et al. Anaplastic thyroid carcinoma with osteoclast-like giant cells. A clinicopathologic, immunohistochemical, and ultrastructural study. Am J Surg Pathol 1991;15:160-8. [PubMed]
- Ordóñez NG, El-Naggar AK, Hickey RC, et al. Anaplastic thyroid carcinoma. Immunocytochemical study of 32 cases. Am J Clin Pathol 1991;96:15-24. [PubMed]
- Yoshida A, Kamma H, Asaga T, et al. Proliferative activity in thyroid tumors. Cancer 1992;69:2548-52. [PubMed]
- O'Neill JP, Shaha AR. Anaplastic thyroid cancer. Oral Oncol 2013;49:702-6. [PubMed]
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012;22:1104-39. [PubMed]
- Hunt JL, Tometsko M. Molecular evidence of anaplastic transformation in coexisting well-differentiated and anaplastic carcinomas of the thyroid. Am J Surg Pathol 2003;27:1559-64. [PubMed]
- Kadota M, Tamaki Y, Sekimoto M, et al. Loss of heterozygosity on chromosome 16p and 18q in anaplastic thyroid carcinoma. Oncol Rep 2003;10:35-8. [PubMed]
- Kitamura Y, Shimizu K, Tanaka S, et al. Allelotyping of anaplastic thyroid carcinoma: frequent allelic losses on 1q, 9p, 11, 17, 19p, and 22q. Genes Chromosomes Cancer 2000;27:244-51. [PubMed]
- Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer 2009;16:17-44. [PubMed]
- Charles RP, Silva J, Iezza G, et al. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol Cancer Res 2014;12:979-86. [PubMed]
- Sugg SL, Ezzat S, Zheng L, et al. Oncogene profile of papillary thyroid carcinoma. Surgery 1999;125:46-52. [PubMed]
- Pita JM, Figueiredo IF, Moura MM, et al. Cell cycle deregulation and TP53 and RAS mutations are major events in poorly differentiated and undifferentiated thyroid carcinomas. J Clin Endocrinol Metab 2014;99:E497-507. [PubMed]
- Santarpia L, El-Naggar AK, Cote GJ, et al. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab 2008;93:278-84. [PubMed]
- García-Rostán G, Costa AM, Pereira-Castro I, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res 2005;65:10199-207. [PubMed]
- Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 2007;13:1161-70. [PubMed]
- Kondo T, Nakazawa T, Murata SI, et al. Expression of CD73 and its ecto-5'-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology 2006;48:612-4. [PubMed]
- Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003;88:5399-404. [PubMed]
- Moretti F, Nanni S, Farsetti A, et al. Effects of exogenous p53 transduction in thyroid tumor cells with different p53 status. J Clin Endocrinol Metab 2000;85:302-8. [PubMed]
- Garcia-Rostan G, Camp RL, Herrero A, et al. Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 2001;158:987-96. [PubMed]
- Wiseman SM, Masoudi H, Niblock P, et al. Derangement of the E-cadherin/catenin complex is involved in transformation of differentiated to anaplastic thyroid carcinoma. Am J Surg 2006;191:581-7. [PubMed]
- Reddi HV, Driscoll CB, Madde P, et al. Redifferentiation and induction of tumor suppressors miR-122 and miR-375 by the PAX8/PPARγ fusion protein inhibits anaplastic thyroid cancer: a novel therapeutic strategy. Cancer Gene Ther 2013;20:267-75. [PubMed]
- Zhang Z, Liu ZB, Ren WM, et al. The miR-200 family regulates the epithelial-mesenchymal transition induced by EGF/EGFR in anaplastic thyroid cancer cells. Int J Mol Med 2012;30:856-62. [PubMed]
- Bauman ME, Tao LC. Cytopathology of papillary carcinoma of the thyroid with anaplastic transformation. A case report. Acta Cytol 1995;39:525-9. [PubMed]
- Poisson T, Deandreis D, Leboulleux S, et al. 18F-fluorodeoxyglucose positron emission tomography and computed tomography in anaplastic thyroid cancer. Eur J Nucl Med Mol Imaging 2010;37:2277-85. [PubMed]
- Hölting T, Meybier H, Buhr H. Problems of tracheotomy in locally invasive anaplastic thyroid cancer. Langenbecks Arch Chir 1989;374:72-6. [PubMed]
- McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 2001;130:1028-34. [PubMed]
- Passler C, Scheuba C, Prager G, et al. Anaplastic (undifferentiated) thyroid carcinoma (ATC). A retrospective analysis. Langenbecks Arch Surg 1999;384:284-93. [PubMed]
- Sugitani I, Miyauchi A, Sugino K, et al. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg 2012;36:1247-54. [PubMed]
- Nilsson O, Lindeberg J, Zedenius J, et al. Anaplastic giant cell carcinoma of the thyroid gland: treatment and survival over a 25-year period. World J Surg 1998;22:725-30. [PubMed]
- Swaak-Kragten AT, de Wilt JH, Schmitz PI, et al. Multimodality treatment for anaplastic thyroid carcinoma--treatment outcome in 75 patients. Radiother Oncol 2009;92:100-4. [PubMed]
- Bhatia A, Rao A, Ang KK, et al. Anaplastic thyroid cancer: Clinical outcomes with conformal radiotherapy. Head Neck 2010;32:829-36. [PubMed]
- De Crevoisier R, Baudin E, Bachelot A, et al. Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J Radiat Oncol Biol Phys 2004;60:1137-43. [PubMed]
- Sosa JA, Balkissoon J, Lu SP, et al. Thyroidectomy followed by fosbretabulin (CA4P) combination regimen appears to suggest improvement in patient survival in anaplastic thyroid cancer. Surgery 2012;152:1078-87. [PubMed]
- Tennvall J, Lundell G, Wahlberg P, et al. Anaplastic thyroid carcinoma: three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. Br J Cancer 2002;86:1848-53. [PubMed]
- Haymart MR, Banerjee M, Yin H, et al. Marginal treatment benefit in anaplastic thyroid cancer. Cancer 2013;119:3133-9. [PubMed]
- Sosa JA, Elisei R, Jarzab B, et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 2014;24:232-40. [PubMed]
- Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013;23:600-4. [PubMed]
- Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med 2013;368:684-5. [PubMed]
- Wagle N, Grabiner BC, Van Allen EM, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med 2014;371:1426-33. [PubMed]
- Kihara M, Miyauchi A, Yamauchi A, et al. Prognostic factors of anaplastic thyroid carcinoma. Surg Today 2004;34:394-8. [PubMed]
- Kim TY, Kim KW, Jung TS, et al. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck 2007;29:765-72. [PubMed]
- Sugitani I, Kasai N, Fujimoto Y, et al. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg 2001;25:617-22. [PubMed]
- Akaishi J, Sugino K, Kitagawa W, et al. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid 2011;21:1183-9. [PubMed]